Abstract
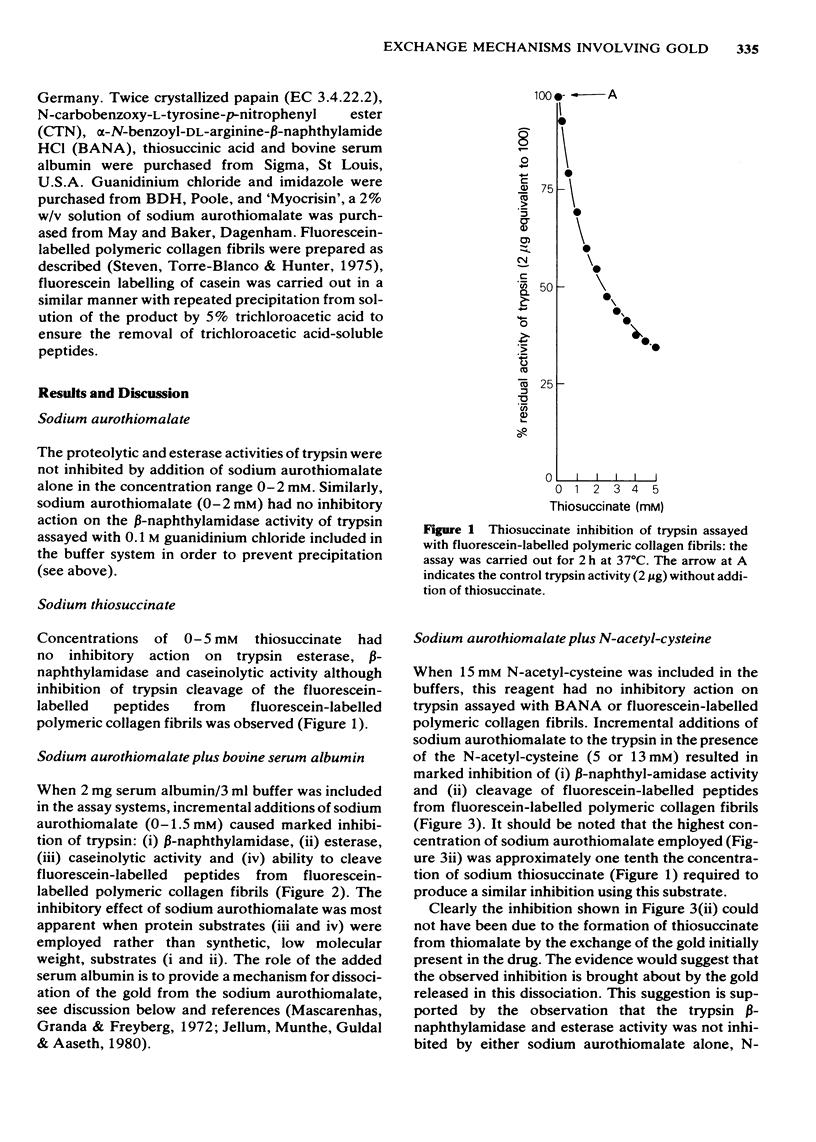
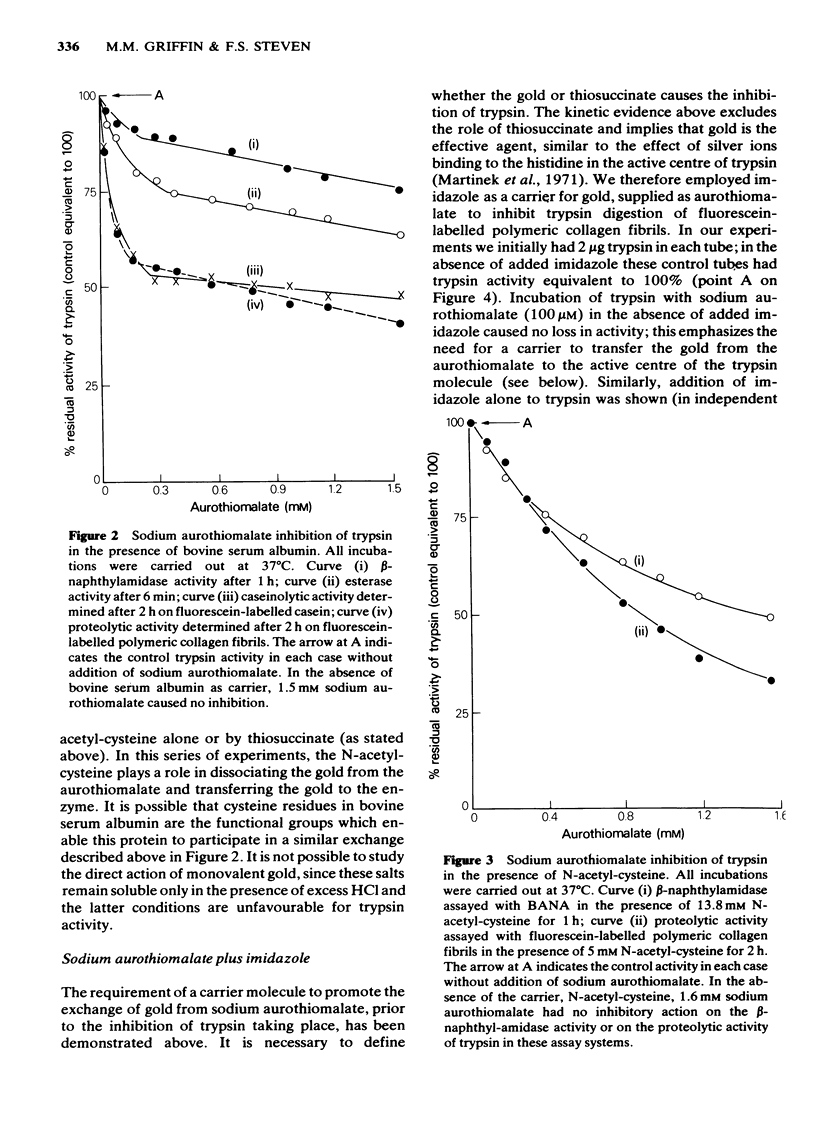
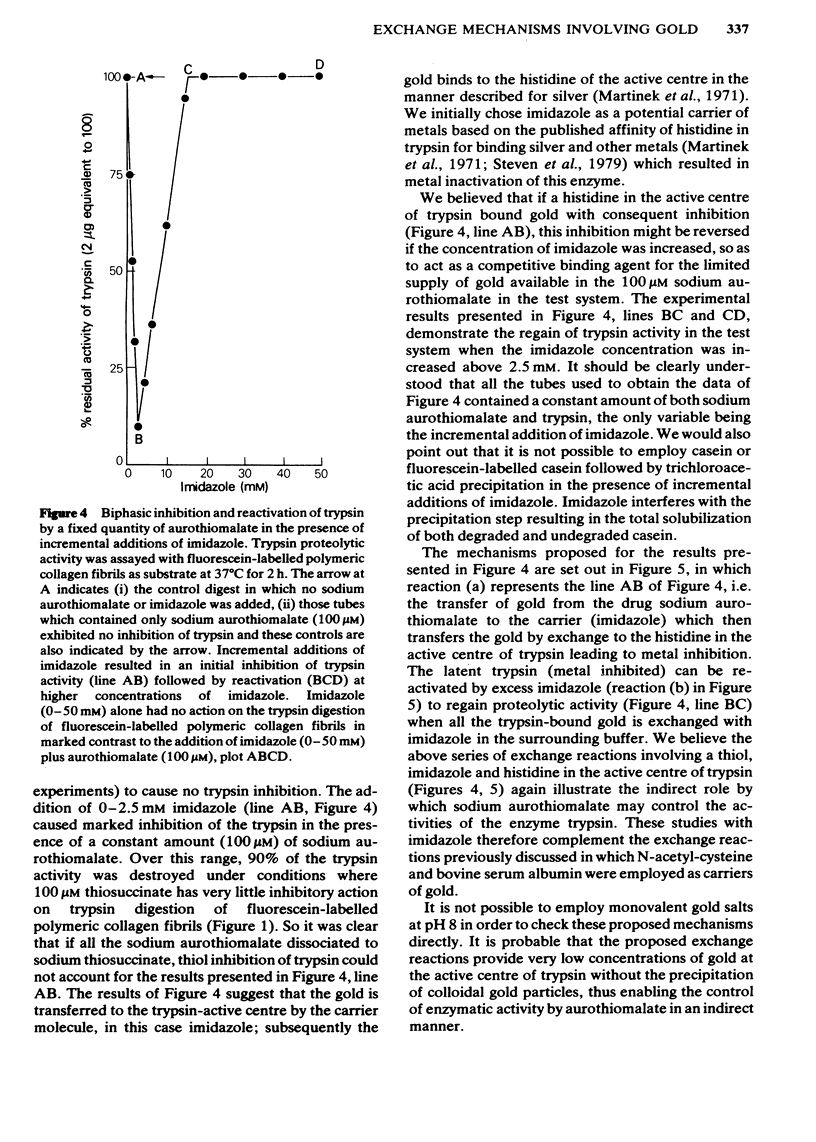
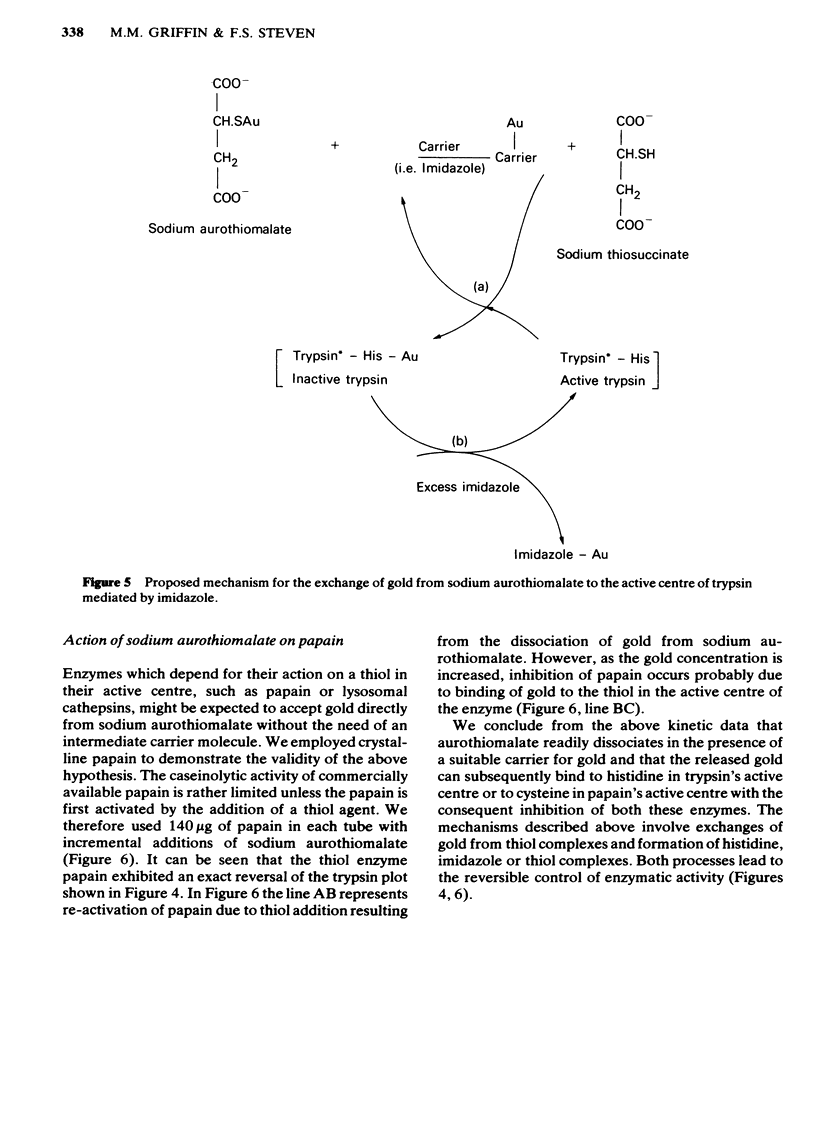
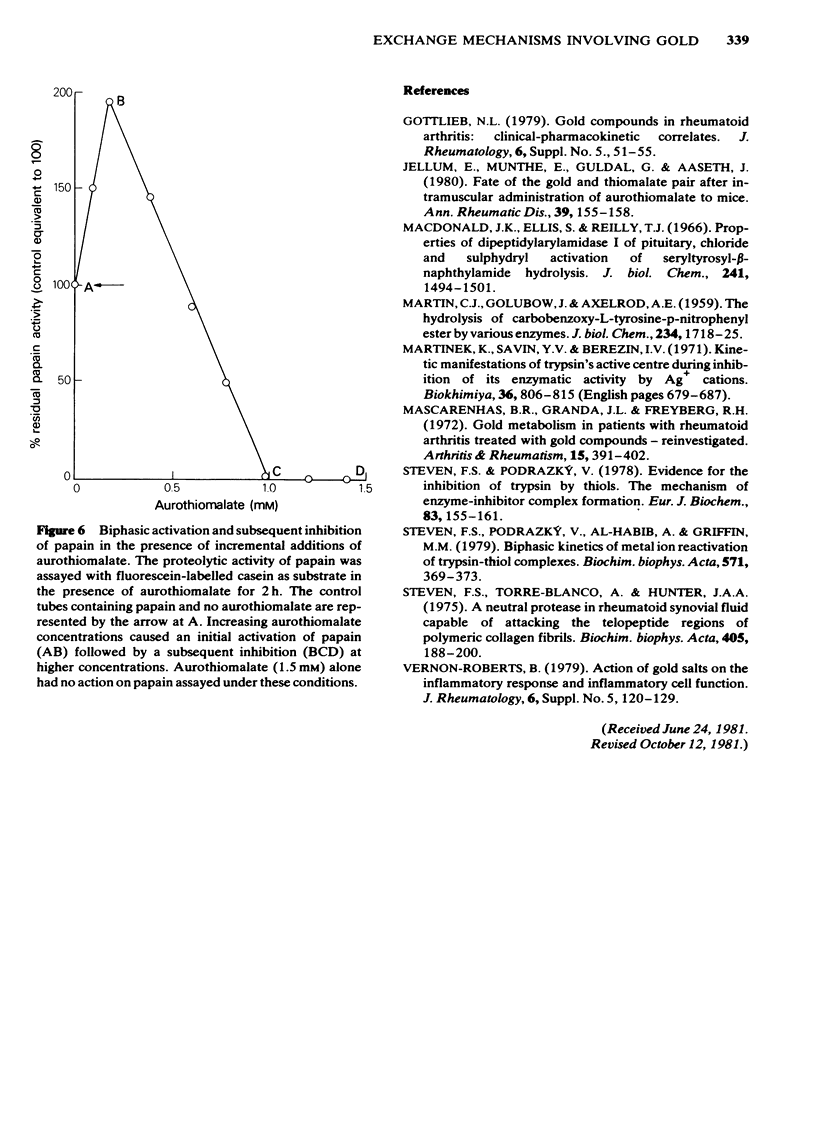
Sodium aurothiomalate has been shown to participate in exchange reactions leading to the inhibition of trypsin; for this exchange to take place it was necessary to include in the test system a suitable thiol, such as N-acetyl-cysteine. Neither N-acetyl-cysteine nor aurothiomalate on their own had any inhibitory action on trypsin. The results indicate that aurothiomalate dissociates in the presence of a carrier to form thiosuccinate and gold. The gold is responsible for trypsin inhibition since independent experiments demonstrated that the total concentration of thiosuccinate was insufficient to cause the observed inhibition of trypsin. Bovine serum albumin was shown to act as a carrier in place of N-acetyl-cysteine. It is known that histidine in the active centre of trypsin binds heavy metal ions with consequent inhibition of the enzyme. In this study, imidazole was shown to act as a carrier for gold from aurothiomalate to trypsin resulting in inhibition. This inhibition by gold was reversed when higher concentrations of imidazole were added to the test system due to competition for the trypsin-bound gold by imidazole. Conversely, the thiol enzyme papain was re-activated in the presence of low concentrations of sodium aurothiomalate and inhibited by higher concentrations of this reagent in a biphasic manner. This observation will be discussed in relation to the dissociation of sodium aurothiomalate. These observations can also be explained in terms of exchange reactions involving thiols and free metal ions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gottlieb N. L. Gold compounds in rheumatoid arthritis: clinical-pharmacokinetic correlates. J Rheumatol Suppl. 1979;5:51–55. [PubMed] [Google Scholar]
- Jellum E., Munthe E., Guldal G., Aaseth J. Fate of the gold and the thiomalate part after intramuscular administration of aurothiomalate to mice. Ann Rheum Dis. 1980 Apr;39(2):155–158. doi: 10.1136/ard.39.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN C. J., GOLUBOW J., AXELROD A. E. The hydrolysis of carbobenzoxy-L-tyrosine p-nitrophenyl ester by various enzymes. J Biol Chem. 1959 Jul;234(7):1718–1725. [PubMed] [Google Scholar]
- Mascarenhas B. R., Granda J. L., Freyberg R. H. Gold metabolism in patients with rheumatoid arthritis treated with gold compounds--reinvestigated. Arthritis Rheum. 1972 Jul-Aug;15(4):391–402. doi: 10.1002/art.1780150410. [DOI] [PubMed] [Google Scholar]
- McDonald J. K., Ellis S., Reilly T. J. Properties of dipeptidyl arylamidase I of the pituitary. Chloride and sulfhydryl activation of seryltyrosyl-beta-naphthylamide hydrolysis. J Biol Chem. 1966 Apr 10;241(7):1494–1501. [PubMed] [Google Scholar]
- Steven F. S., Podrazký V., Al-Habib A., Griffin M. M. Biphasic kinetics of metal ion reactivation of trypsin-thiol complexes. Biochim Biophys Acta. 1979 Dec 7;571(2):369–373. doi: 10.1016/0005-2744(79)90107-4. [DOI] [PubMed] [Google Scholar]
- Steven F. S., Podrazký V. Evidence for the inhibition of trypsin by thiols. The mechanism of enzyme-inhibitor complex formation. Eur J Biochem. 1978 Feb 1;83(1):155–161. doi: 10.1111/j.1432-1033.1978.tb12079.x. [DOI] [PubMed] [Google Scholar]
- Steven F. S., Torre-Blanco A., Hunter J. A. A neutral protease in rheumatoid synovial fluid capable of attacking the telopeptide regions of polymeric collagen fibrils. Biochim Biophys Acta. 1975 Sep 9;405(1):188–200. doi: 10.1016/0005-2795(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Vernon-Roberts B. Action of gold salts on the inflammatory response and inflammatory cell function. J Rheumatol Suppl. 1979;5:120–129. [PubMed] [Google Scholar]


