Abstract
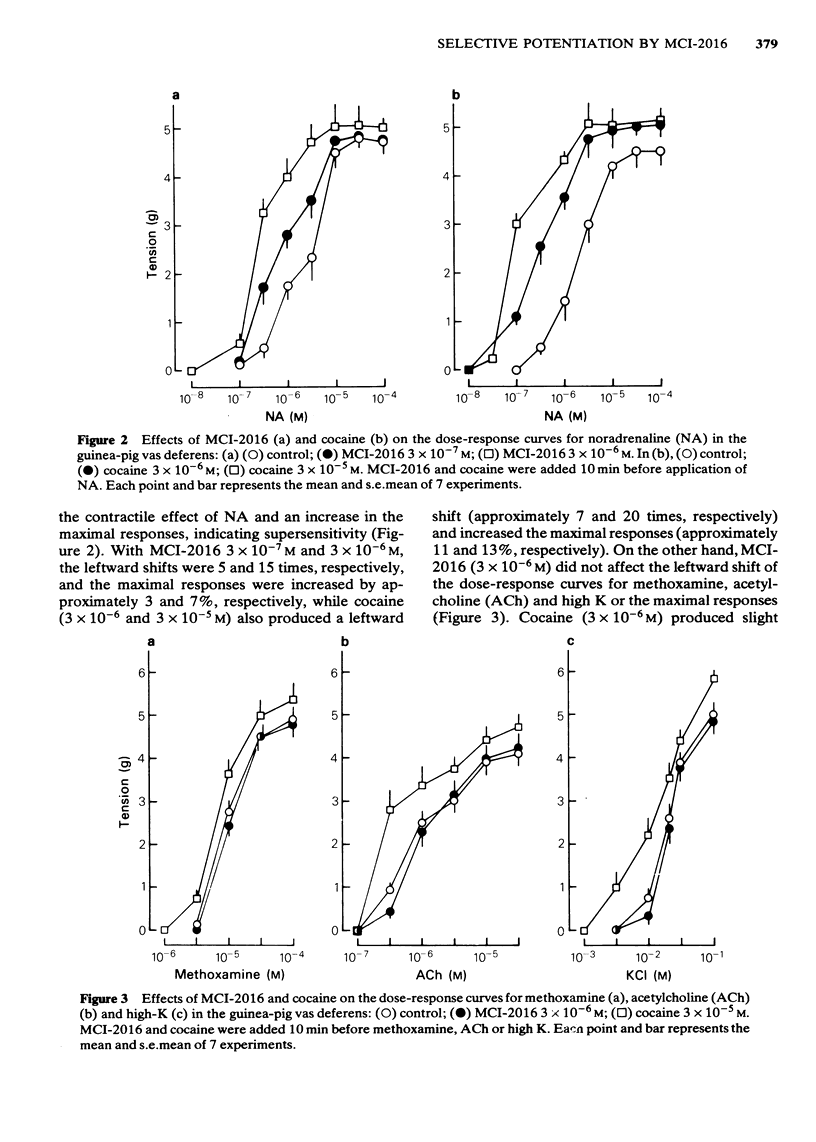
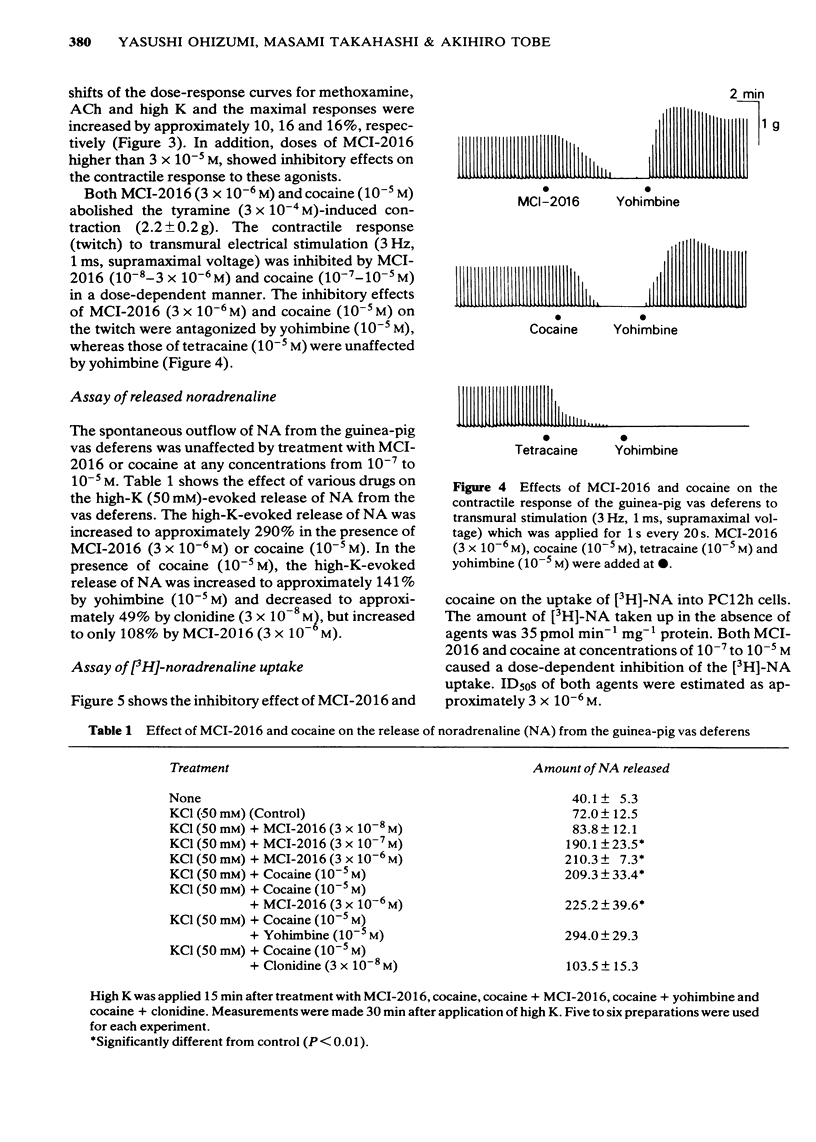
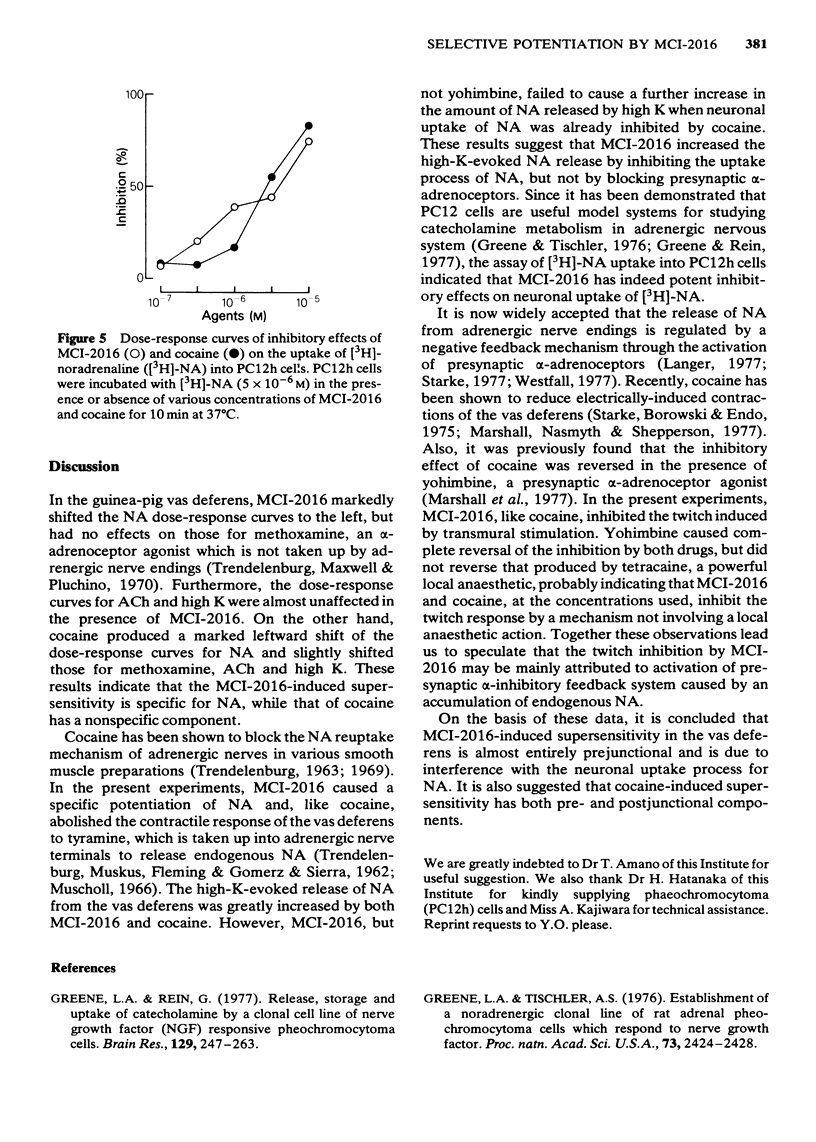
In the isolated vas deferens of the guinea-pig, the effects of 2-(4-methylaminobutoxy) diphenylmethane hydrochloride (MCI-2016), a new psychotropic drug, on the contractile response to various agonists or transmural electrical stimulation and on the release of noradrenaline (NA) from the tissue were examined and compared with cocaine. MCI-2016 (3 X 10(-6)M) and cocaine (3 X 10(-5)M) produced a leftward shift (15 and 20 times, respectively) of the dose-response curves for the contractile effect of NA and increased the maximum contractile response to NA by approximately 7 and 14% respectively. MCI-2016 had no apparent effect on the dose-response curves for methoxamine, acetylcholine and high K, while cocaine markedly shifted those for these agents to the left and increased the maximal responses (10, 16 and 16%, respectively). MCI-2016 and cocaine abolished the tyramine (3 X 10(-4)M)-induced contraction and inhibited the twitch response to transmural electrical stimulation in a dose-dependent manner. The inhibitory effects of both drugs on the twitch were reversed by yohimbine (10(-5)M). The spontaneous outflow of NA from the vas deferens was unaffected by MCI-2016 (3 X 10(-6)M) and cocaine (10(-5)M), while the high-K-evoked release of NA was increased by both drugs. In the presence of cocaine (10(-5)M), the high-K-evoked release of NA was markedly increased by yohimbine (10(-5)M) and decreased by clonidine (3 X 10(-8)M), but only slightly increased by MCI-2016 (3 X 10(-6)M).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greene L. A., Rein G. Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheo-chromocytoma cells. Brain Res. 1977 Jul 1;129(2):247–263. doi: 10.1016/0006-8993(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka H. Nerve growth factor-mediated stimulation of tyrosine hydroxylase activity in a clonal rat pheochromocytoma cell line. Brain Res. 1981 Oct 19;222(2):225–233. doi: 10.1016/0006-8993(81)91029-5. [DOI] [PubMed] [Google Scholar]
- Kikumoto R., Tobe A., Tonomura S. Synthesis and antidepressant activity of substituted (omega-aminoalkoxy)benzene derivatives. J Med Chem. 1981 Feb;24(2):145–148. doi: 10.1021/jm00134a004. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscholl E. Modification of sympathetic function. Indirectly acting sympathomimetic amines. Pharmacol Rev. 1966 Mar;18(1):551–559. [PubMed] [Google Scholar]
- Ohizumi Y., Shibata S. Mechanism of the excitatory action of palytoxin and N-acetylpalytoxin in the isolated guinea-pig vas deferens. J Pharmacol Exp Ther. 1980 Jul;214(1):209–212. [PubMed] [Google Scholar]
- Starke K., Borowski E., Endo T. Preferential blockade of presynaptic alpha-adrenoceptors by yohimbine. Eur J Pharmacol. 1975 Dec;34(2):385–388. doi: 10.1016/0014-2999(75)90268-x. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U., MUSKUS A., FLEMING W. W., de la GOMEZ ALONSO SIERRA B. Effect of cocaine, denervation and decentralization on the response of the nictitating membrane to various sympathomimetic amines. J Pharmacol Exp Ther. 1962 Nov;138:181–193. [PubMed] [Google Scholar]
- TRENDELENBURG U. Supersensitivity and subsensitivity to sympathomimetic amines. Pharmacol Rev. 1963 Jun;15:225–276. [PubMed] [Google Scholar]
- Tobe A., Yoshida Y., Ikoma H., Tonomura S., Kikumoto R. Pharmacological evaluation of 2-(4-methylaminobutoxy)diphenylmethane hydrochloride (MCI-2016), a new psychotropic drug with antidepressant activity. Arzneimittelforschung. 1981;31(8):1278–1285. [PubMed] [Google Scholar]
- Trendelenburg U., Maxwell R. A., Pluchino S. Methoxamine as a tool to assess the importance of intraneuronal uptake of l-norepinephrine in the cat's nictitating membrane. J Pharmacol Exp Ther. 1970 Mar;172(1):91–99. [PubMed] [Google Scholar]
- Westfall T. C. Local regulation of adrenergic neurotransmission. Physiol Rev. 1977 Oct;57(4):659–728. doi: 10.1152/physrev.1977.57.4.659. [DOI] [PubMed] [Google Scholar]


