Abstract
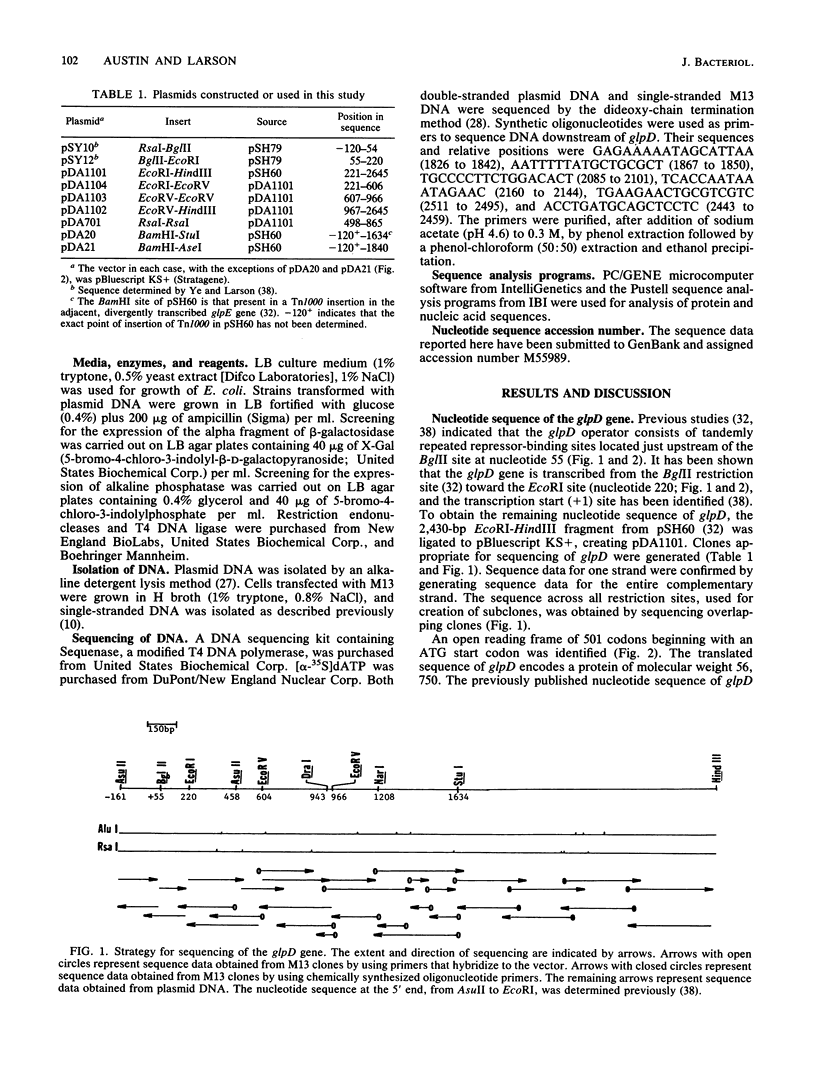
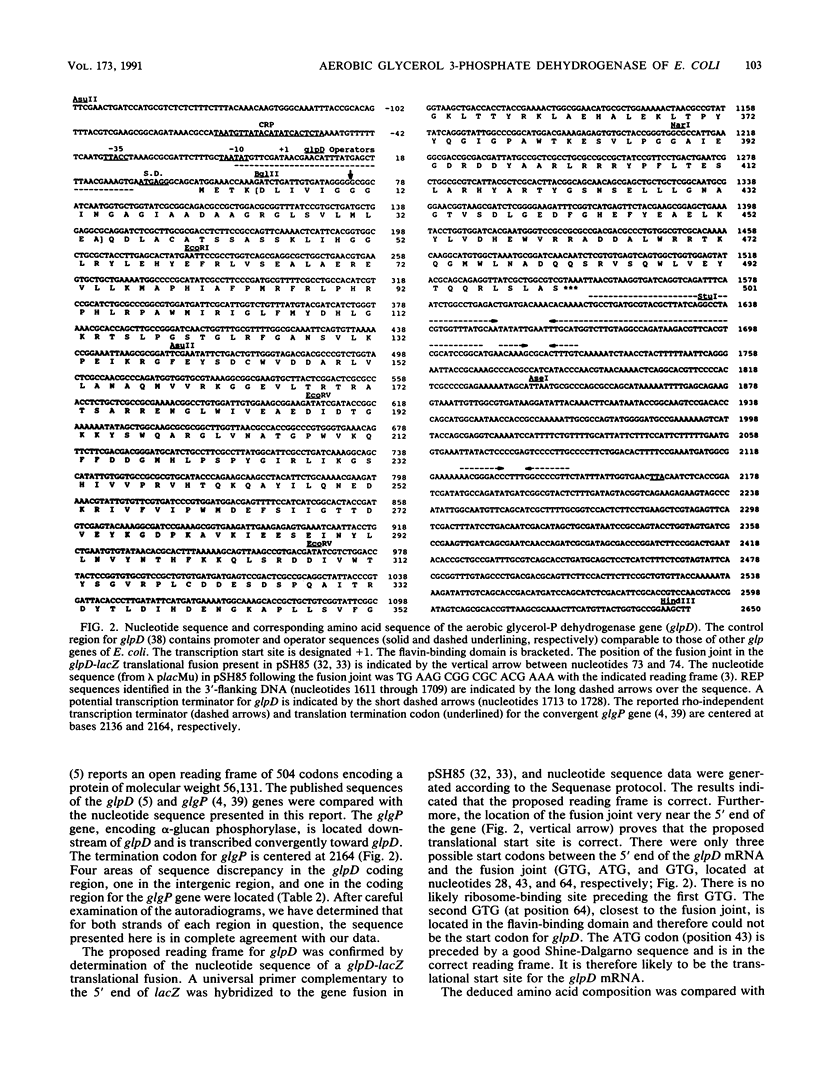
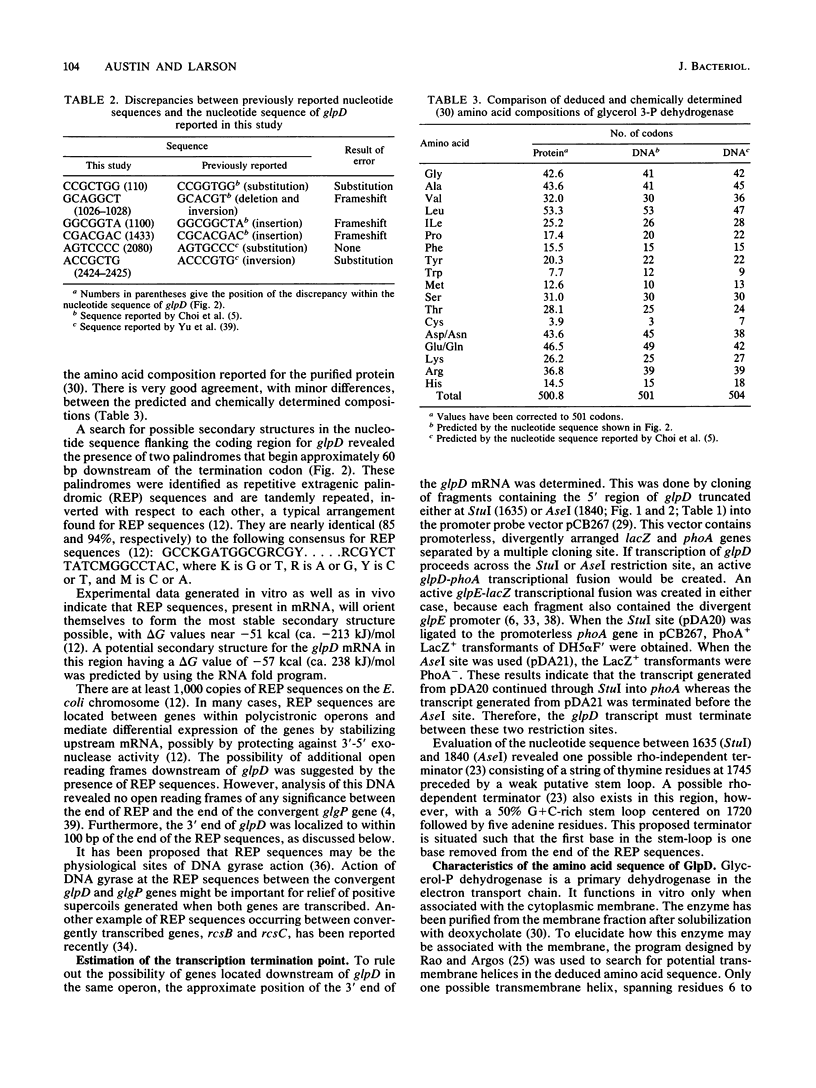
Aerobic sn-glycerol 3-phosphate dehydrogenase, encoded by the glpD gene of Escherichia coli, is a cytoplasmic membrane-associated respiratory enzyme. The nucleotide sequence of glpD was determined. An open reading frame of 501 codons was preceded by a consensus Shine-Dalgarno sequence. The proposed translational start and reading frame of glpD were confirmed by determining the nucleotide sequence across the fusion joint of a glpD-lacZ translational fusion. The predicted molecular weight, 56,750, corresponds well with the reported value of 58,000 for purified sn-glycerol 3-phosphate dehydrogenase. The flavin-binding domain, located at the amino terminus, was identified by comparison with the amino acid sequences of other flavoproteins from E. coli. Repetitive extragenic palindromic sequences were identified downstream of the glpD coding region. The site for transcription termination was located between 87 and 216 bp downstream of the translation stop codon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T. C., Davenport R. C., Jr, Giammona D. A., Lolis E., Petsko G. A., Ringe D. Crystallography and site-directed mutagenesis of yeast triosephosphate isomerase: what can we learn about catalysis from a "simple" enzyme? Cold Spring Harb Symp Quant Biol. 1987;52:603–613. doi: 10.1101/sqb.1987.052.01.069. [DOI] [PubMed] [Google Scholar]
- Bilofsky H. S., Burks C. The GenBank genetic sequence data bank. Nucleic Acids Res. 1988 Mar 11;16(5):1861–1863. doi: 10.1093/nar/16.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weisemann J. M., Weinstock G. M. Lambda placMu: a transposable derivative of bacteriophage lambda for creating lacZ protein fusions in a single step. J Bacteriol. 1984 Jun;158(3):1084–1093. doi: 10.1128/jb.158.3.1084-1093.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. L., Kawamukai M., Utsumi R., Sakai H., Komano T. Molecular cloning and sequencing of the glycogen phosphorylase gene from Escherichia coli. FEBS Lett. 1989 Jan 30;243(2):193–198. doi: 10.1016/0014-5793(89)80128-0. [DOI] [PubMed] [Google Scholar]
- Choi Y. L., Kawase S., Nishida T., Sakai H., Komano T., Kawamukai M., Utsumi R., Kohara Y., Akiyama K. Nucleotide sequence of the glpR gene encoding the repressor for the glycerol-3-phosphate regulon of Escherichia coli K12. Nucleic Acids Res. 1988 Aug 11;16(15):7732–7732. doi: 10.1093/nar/16.15.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T., Eiglmeier K., Ahmed S., Honore N., Elmes L., Anderson W. F., Weiner J. H. Nucleotide sequence and gene-polypeptide relationships of the glpABC operon encoding the anaerobic sn-glycerol-3-phosphate dehydrogenase of Escherichia coli K-12. J Bacteriol. 1988 Jun;170(6):2448–2456. doi: 10.1128/jb.170.6.2448-2456.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann M., Boos W., Ormseth E., Schweizer H., Larson T. J. Divergent transcription of the sn-glycerol-3-phosphate active transport (glpT) and anaerobic sn-glycerol-3-phosphate dehydrogenase (glpA glpC glpB) genes of Escherichia coli K-12. J Bacteriol. 1987 Feb;169(2):526–532. doi: 10.1128/jb.169.2.526-532.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Gutfinger T. cDNA sequence of adrenodoxin reductase. Identification of NADP-binding sites in oxidoreductases. Eur J Biochem. 1989 Mar 15;180(2):479–484. doi: 10.1111/j.1432-1033.1989.tb14671.x. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., McLaren R. S., Newbury S. F. Repetitive extragenic palindromic sequences, mRNA stability and gene expression: evolution by gene conversion? A review. Gene. 1988 Dec 10;72(1-2):3–14. doi: 10.1016/0378-1119(88)90122-9. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Cameron D. C., Lin E. C. A second global regulator gene (arcB) mediating repression of enzymes in aerobic pathways of Escherichia coli. J Bacteriol. 1989 Feb;171(2):868–873. doi: 10.1128/jb.171.2.868-873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Cole S. T., Lin E. C. Multiple regulatory elements for the glpA operon encoding anaerobic glycerol-3-phosphate dehydrogenase and the glpD operon encoding aerobic glycerol-3-phosphate dehydrogenase in Escherichia coli: further characterization of respiratory control. J Bacteriol. 1990 Jan;172(1):179–184. doi: 10.1128/jb.172.1.179-184.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Ye S. Z., Weissenborn D. L., Hoffmann H. J., Schweizer H. Purification and characterization of the repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K12. J Biol Chem. 1987 Nov 25;262(33):15869–15874. [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Muramatsu S., Mizuno T. Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989 Jun 12;17(11):4378–4378. [PMC free article] [PubMed] [Google Scholar]
- Paul C., Rosenbusch J. P. Folding patterns of porin and bacteriorhodopsin. EMBO J. 1985 Jun;4(6):1593–1597. doi: 10.1002/j.1460-2075.1985.tb03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew D. W., Ma D. P., Conrad C. A., Johnson J. R. Escherichia coli glycerol kinase. Cloning and sequencing of the glpK gene and the primary structure of the enzyme. J Biol Chem. 1988 Jan 5;263(1):135–139. [PubMed] [Google Scholar]
- Pichersky E., Gottlieb L. D., Hess J. F. Nucleotide sequence of the triose phosphate isomerase gene of Escherichia coli. Mol Gen Genet. 1984;195(1-2):314–320. doi: 10.1007/BF00332765. [DOI] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., McElvany K. D., Borders C. L., Jr Arginyl residues: anion recognition sites in enzymes. Science. 1977 Mar 4;195(4281):884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Beck C. F. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene. 1986;42(1):37–48. doi: 10.1016/0378-1119(86)90148-4. [DOI] [PubMed] [Google Scholar]
- Schryvers A., Lohmeier E., Weiner J. H. Chemical and functional properties of the native and reconstituted forms of the membrane-bound, aerobic glycerol-3-phosphate dehydrogenase of Escherichia coli. J Biol Chem. 1978 Feb 10;253(3):783–788. [PubMed] [Google Scholar]
- Schweizer H., Boos W., Larson T. J. Repressor for the sn-glycerol-3-phosphate regulon of Escherichia coli K-12: cloning of the glpR gene and identification of its product. J Bacteriol. 1985 Feb;161(2):563–566. doi: 10.1128/jb.161.2.563-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Larson T. J. Cloning and characterization of the aerobic sn-glycerol-3-phosphate dehydrogenase structural gene glpD of Escherichia coli K-12. J Bacteriol. 1987 Feb;169(2):507–513. doi: 10.1128/jb.169.2.507-513.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Sweet G., Larson T. J. Physical and genetic structure of the glpD-malT interval of the Escherichia coli K-12 chromosome. Identification of two new structural genes of the glp-regulon. Mol Gen Genet. 1986 Mar;202(3):488–492. doi: 10.1007/BF00333282. [DOI] [PubMed] [Google Scholar]
- Stout V., Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990 Feb;172(2):659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet G., Gandor C., Voegele R., Wittekindt N., Beuerle J., Truniger V., Lin E. C., Boos W. Glycerol facilitator of Escherichia coli: cloning of glpF and identification of the glpF product. J Bacteriol. 1990 Jan;172(1):424–430. doi: 10.1128/jb.172.1.424-430.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ames G. F. DNA gyrase binds to the family of prokaryotic repetitive extragenic palindromic sequences. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8850–8854. doi: 10.1073/pnas.85.23.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Ye S. Z., Larson T. J. Structures of the promoter and operator of the glpD gene encoding aerobic sn-glycerol-3-phosphate dehydrogenase of Escherichia coli K-12. J Bacteriol. 1988 Sep;170(9):4209–4215. doi: 10.1128/jb.170.9.4209-4215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Jen Y., Takeuchi E., Inouye M., Nakayama H., Tagaya M., Fukui T. Alpha-glucan phosphorylase from Escherichia coli. Cloning of the gene, and purification and characterization of the protein. J Biol Chem. 1988 Sep 25;263(27):13706–13711. [PubMed] [Google Scholar]



