Abstract
1 A modification of the abdominal constriction test in mice has been developed, and used to study the antinociceptive effects of morphine and several related drugs. In most experiments, acetic acid (0.6% i.p.) was used as the nociceptive stimulus, and in a few cases, acetylcholine (3.2 mg/kg i.p.) was used. When the abdominal constriction response had reached a maximum, the drugs under test were given intraperitoneally, and their ability to decrease the number of abdominal constrictions was determined, beginning immediately after its administration. The aim of this study was to investigate the possibility that morphine and its congeners may produce an antinociceptive effect by an action within the peritoneum.
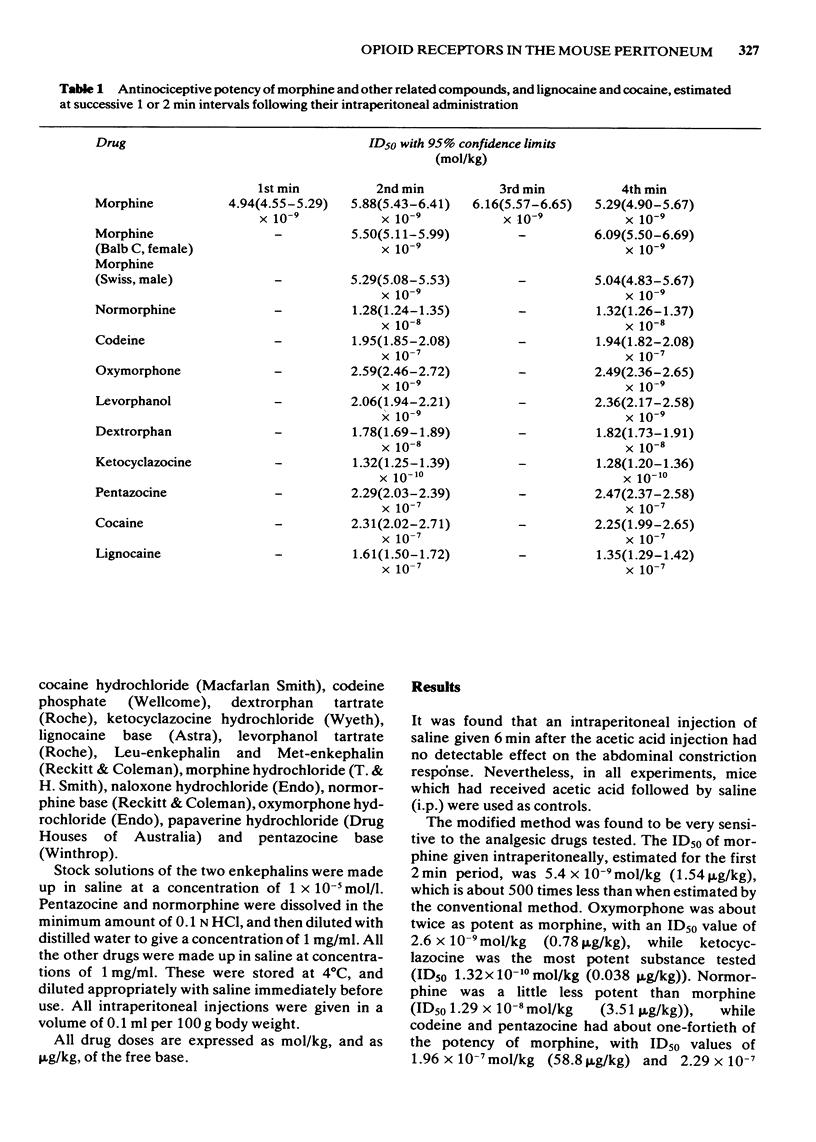
2 It was found that morphine was an extremely potent antinociceptive agent in this modified test, with an ID50 of 5.4 × 10-9 mol/kg (1.54 μg/kg). Codeine and pentazocine were about 40 times less active and oxymorphine was about twice as potent as morphine. Met- and Leu-enkephalin were also potent but their action diminished very rapidly with time. Ketocyclazocine was the most potent substance tested, and had an ID50 value of 1.26 × 10-10 mol/kg (0.036 μg/kg). All the drugs tested produced their maximal effect within 1 or 2 min of administration.
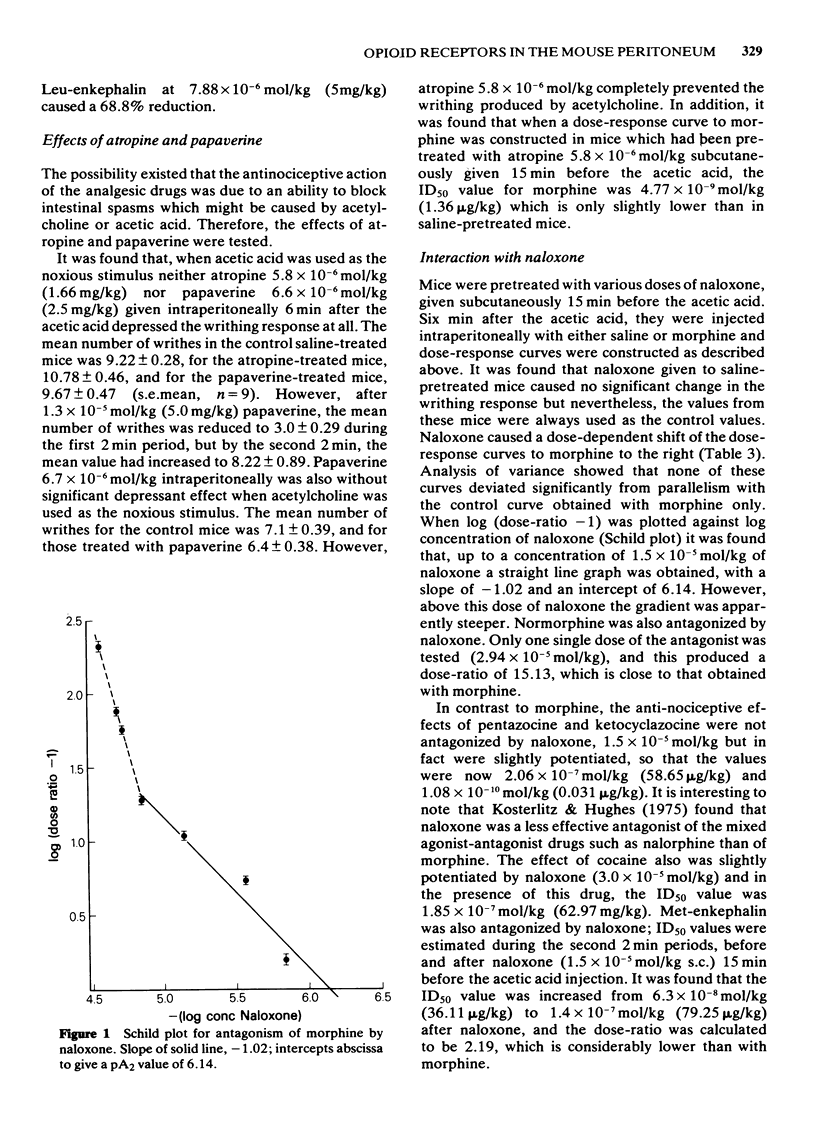
3 Pretreatment of the mice with naloxone caused a dose-dependent shift to the right of the dose-response curve to morphine. The pAx plot was linear over part of the range, with a slope of -1.02 and the `apparent pA2' value was 6.14. Naloxone was much less effective in antagonizing Met-enkephalin, and caused a slight potentiation of ketocyclazocine and pentazocine and of cocaine, which was used for comparison.
4 Pretreatment of mice with morphine, 3 h earlier, caused a marked tolerance to a subsequent dose of morphine, and a potentiation of the antagonist potency of naloxone. However, there was little cross-tolerance between morphine and Leu-enkephalin.
5 It is concluded that morphine and its congeners can produce an antinociceptive effect by an action within the mouse peritoneum, presumably by interacting with one or more types of opioid receptors which may be situated on sensory nerve endings.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley G. A., Copeland I. W., Starr J. The actions of some alpha-adrenoreceptor agonists and antagonists in an antinociceptive test in mice. Clin Exp Pharmacol Physiol. 1977 Jul-Aug;4(4):405–419. doi: 10.1111/j.1440-1681.1977.tb02678.x. [DOI] [PubMed] [Google Scholar]
- Buscher H. H., Hill R. C., Römer D., Cardinaux F., Closse A., Hauser D., Pless J. Evidence for analgesic activity of enkephalin in the mouse. Nature. 1976 Jun 3;261(5559):423–425. doi: 10.1038/261423a0. [DOI] [PubMed] [Google Scholar]
- Clement-Jones V., Lowry P. J., Rees L. H., Besser G. M. Met-enkephalin circulates in human plasma. Nature. 1980 Jan 17;283(5744):295–297. doi: 10.1038/283295a0. [DOI] [PubMed] [Google Scholar]
- Hayashi G., Takemori A. E. The type of analgesic-receptor interaction involved in certain analgesic assays. Eur J Pharmacol. 1971 Sep;16(1):63–66. doi: 10.1016/0014-2999(71)90057-4. [DOI] [PubMed] [Google Scholar]
- Hughes J. Isolation of an endogenous compound from the brain with pharmacological properties similar to morphine. Brain Res. 1975 May 2;88(2):295–308. doi: 10.1016/0006-8993(75)90391-1. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Hughes J. Some thoughts on the significance of enkephalin, the endogenous ligand. Life Sci. 1975 Jul 1;17(1):91–96. doi: 10.1016/0024-3205(75)90243-x. [DOI] [PubMed] [Google Scholar]
- LUDUENA F. P. Experimental spinal anesthesia. Arch Int Pharmacodyn Ther. 1957 Jan 1;109(1-2):143–156. [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- SIEGMUND E., CADMUS R., LU G. A method for evaluating both non-narcotic and narcotic analgesics. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):729–731. doi: 10.3181/00379727-95-23345. [DOI] [PubMed] [Google Scholar]
- Tulunay F. C., Takemori A. E. The increased efficacy of narcotic antagonists induced by various narcotic analgesics. J Pharmacol Exp Ther. 1974 Sep;190(3):395–400. [PubMed] [Google Scholar]
- Waterfield A. A., Hughes J., Kosterlitz H. W. Cross tolerance between morphine and methionine-enkephalin. Nature. 1976 Apr 15;260(5552):624–625. doi: 10.1038/260624a0. [DOI] [PubMed] [Google Scholar]
- Waterfield A. A., Smokcum R. W., Hughes J., Kosterlitz H. W., Henderson G. In vitro pharmacology of the opioid peptides, enkephalins and endorphins. Eur J Pharmacol. 1977 May 15;43(2):107–116. doi: 10.1016/0014-2999(77)90123-6. [DOI] [PubMed] [Google Scholar]


