Abstract
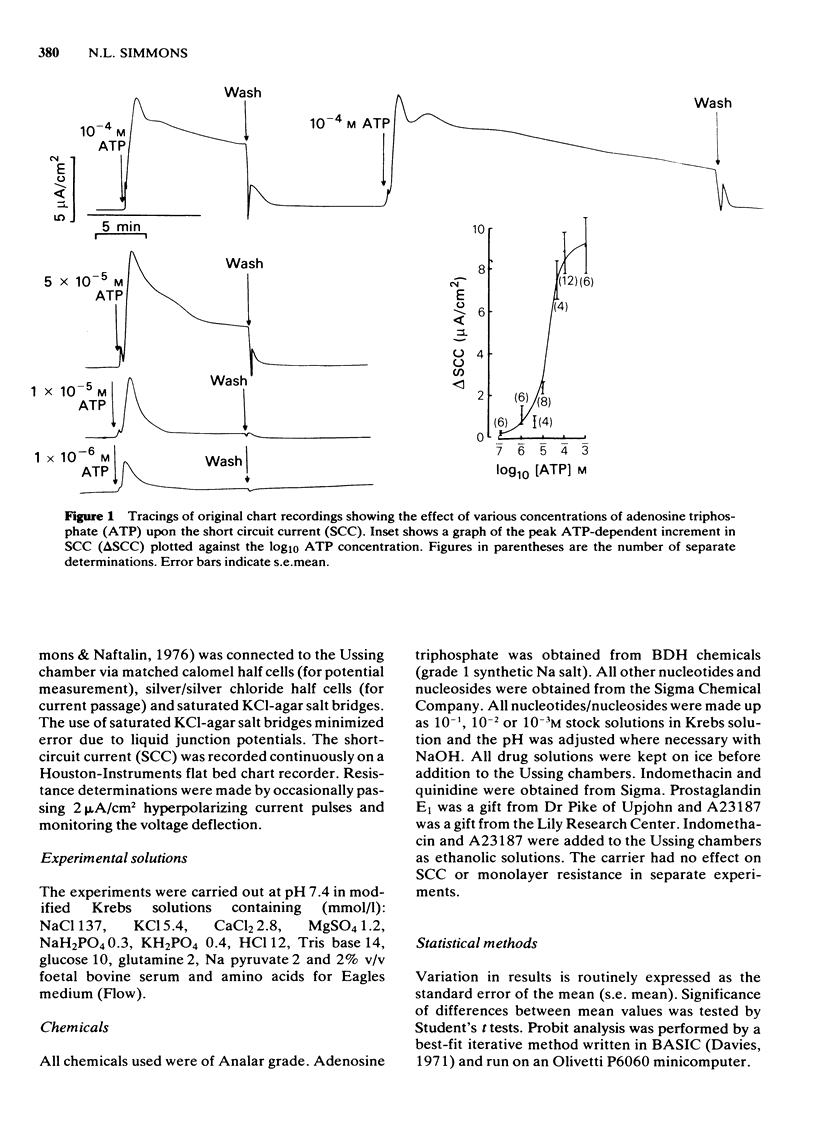
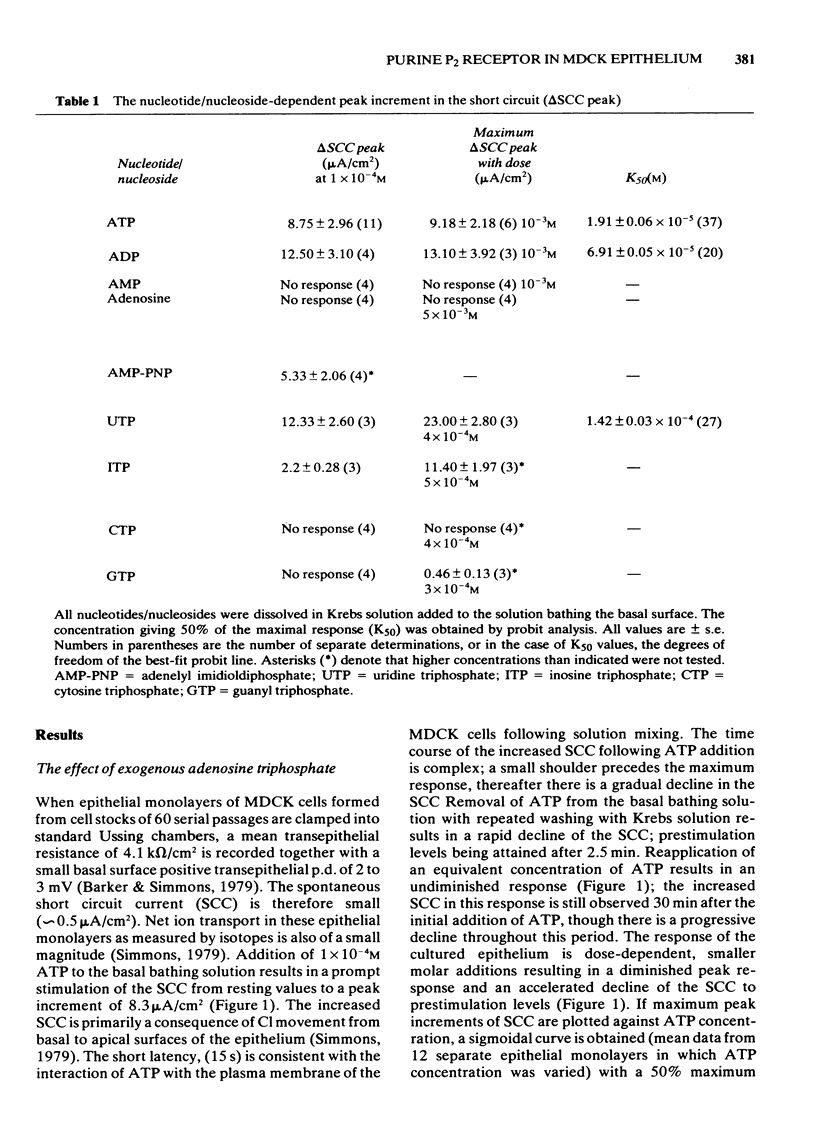
1 Exogenous adenosine triphosphate (ATP) stimulates the short circuit current (SCC) in a cultured renal-derived epithelium (MDCK). Half-maximal stimulation is achieved at 1.91 X 10(-5) M ATP. 2 It is suggested that ATP interacts with a P2 purine receptor upon the basis of (a) agonist potency (ATP greater than adenosine diphosphate much greater than adenosine monophosphate, adenosine; ATP greater than uridine triphosphate greater than inosine triphosphate much greater than cytosine triphosphate, guanosine triphosphate); (b) the inhibition of the ATP response by quinidine (1 X 10(-3) M) but not by theophylline (1 X 10(-3) M). 3 Indomethacin (1 X 10(-5) M) inhibits the response of the cultured epithelium to ATP. 4 Prostaglandin E1 (PGE1) stimulates SCC but potentiates the effect of ATP on SCC. The divalent cationic ionophore A23187 (1 X 10(-6) M) transiently stimulates SCC itself and abolishes ATP-induced stimulation of the SCC.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiton J. F., Lamb J. F. The effect of exogenous adenosine triphosphate on potassium movements in HeLa cells. Q J Exp Physiol Cogn Med Sci. 1980 Jan;65(1):47–62. doi: 10.1113/expphysiol.1980.sp002491. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Barker G., Simmons N. L. Dog kidney cell monolayers can display properties similar to high-resistance epithelia [proceedings]. J Physiol. 1979 Apr;289:33P–34P. [PubMed] [Google Scholar]
- Boyd I. A., Forrester T. The release of adenosine triphosphate from frog skeletal muscle in vitro. J Physiol. 1968 Nov;199(1):115–135. doi: 10.1113/jphysiol.1968.sp008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilundain A., Naftalin R. J. Role of Ca(2+)-dependent regulator protein in intestinal secretion. Nature. 1979 May 31;279(5712):446–448. doi: 10.1038/279446a0. [DOI] [PubMed] [Google Scholar]
- Kohn P. G., Newey H., Smyth D. H. The effect of adenosine triphosphate on the transmural potential in rat small intestine. J Physiol. 1970 May;208(1):203–220. doi: 10.1113/jphysiol.1970.sp009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L. Chemical carcinogens stimulate canine kidney (MDCK) cells to produce prostaglandins. Nature. 1977 Aug 4;268(5619):447–448. doi: 10.1038/268447a0. [DOI] [PubMed] [Google Scholar]
- Liang C. T., Sacktor B. Preparation of renal cortex basal-lateral and bursh border membranes. Localization of adenylate cyclase and guanylate cyclase activities. Biochim Biophys Acta. 1977 May 2;466(3):474–487. doi: 10.1016/0005-2736(77)90340-6. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorive G., Kleinzeller A. The effect of ATP and Ca 2+ on the cell volume in isolated kidney tubules. Biochim Biophys Acta. 1972 Jul 3;274(1):226–239. doi: 10.1016/0005-2736(72)90296-9. [DOI] [PubMed] [Google Scholar]
- Simmons N. L. Ion transport in high-resistance dog-kidney cell monolayers: the effect of adenosine 5', triphosphate [proceedings]. J Physiol. 1979 May;290(2):28P–29P. [PubMed] [Google Scholar]
- Simmons N. L., Naftalin R. J. Bidirectional sodium ion movements via the paracellular and transcellular routes across short-circuited rabbit ileum. Biochim Biophys Acta. 1976 Oct 19;448(3):426–450. doi: 10.1016/0005-2736(76)90298-4. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Westcott K. R., Engelhard V. H., Storm D. R. Production of cyclic AMP from extracellular ATP by intact LM cells. Biochim Biophys Acta. 1979 Feb 19;583(1):47–54. doi: 10.1016/0304-4165(79)90308-8. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


