Abstract
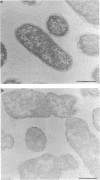
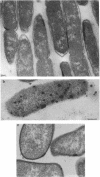
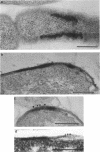
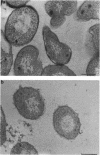
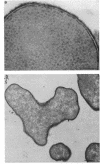
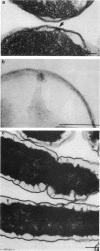
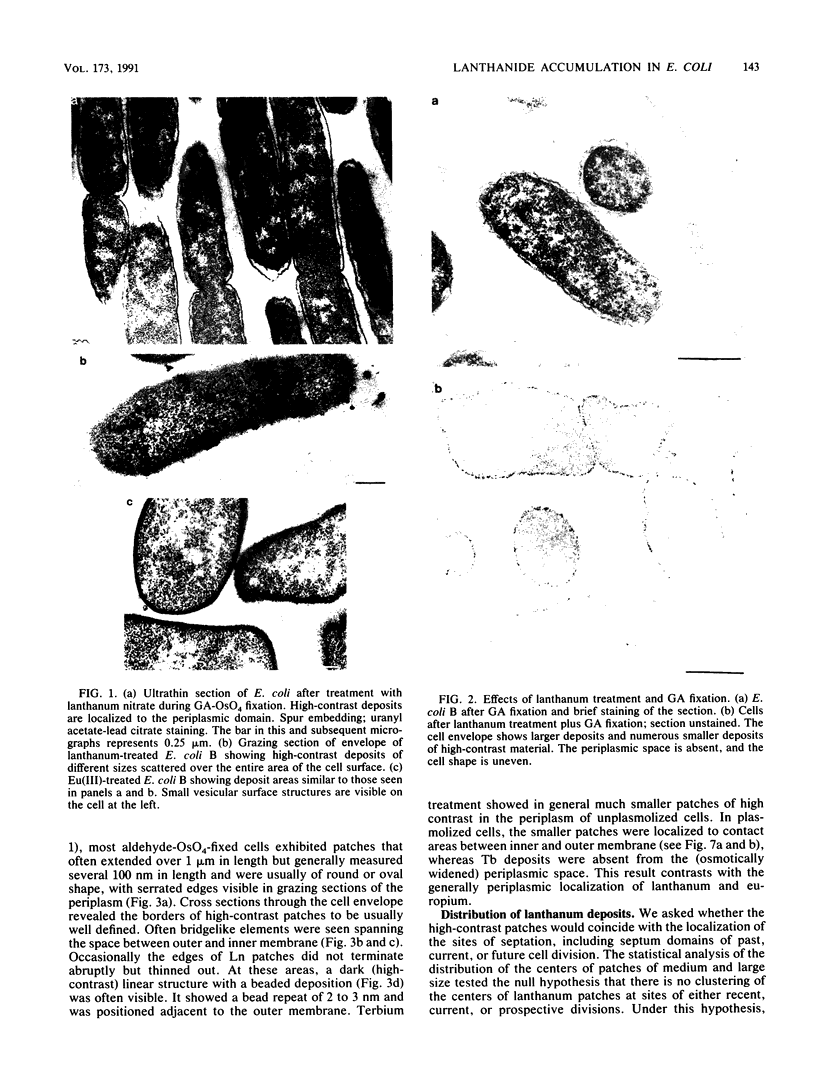
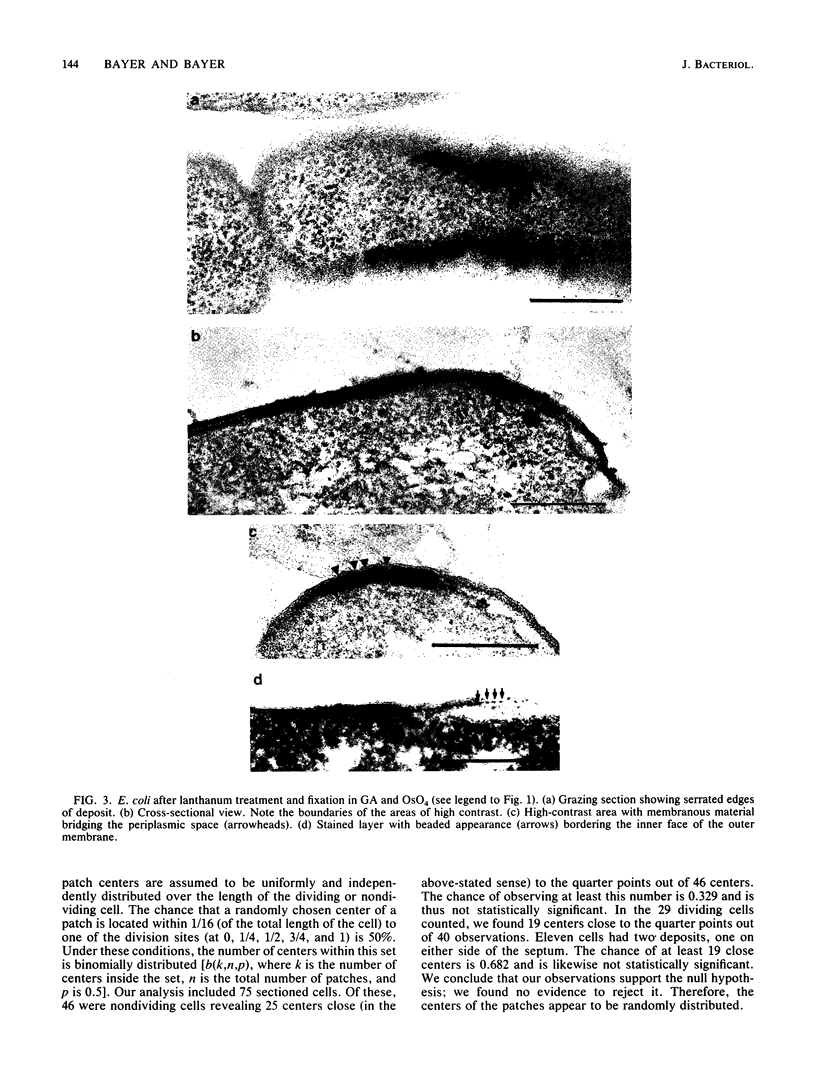
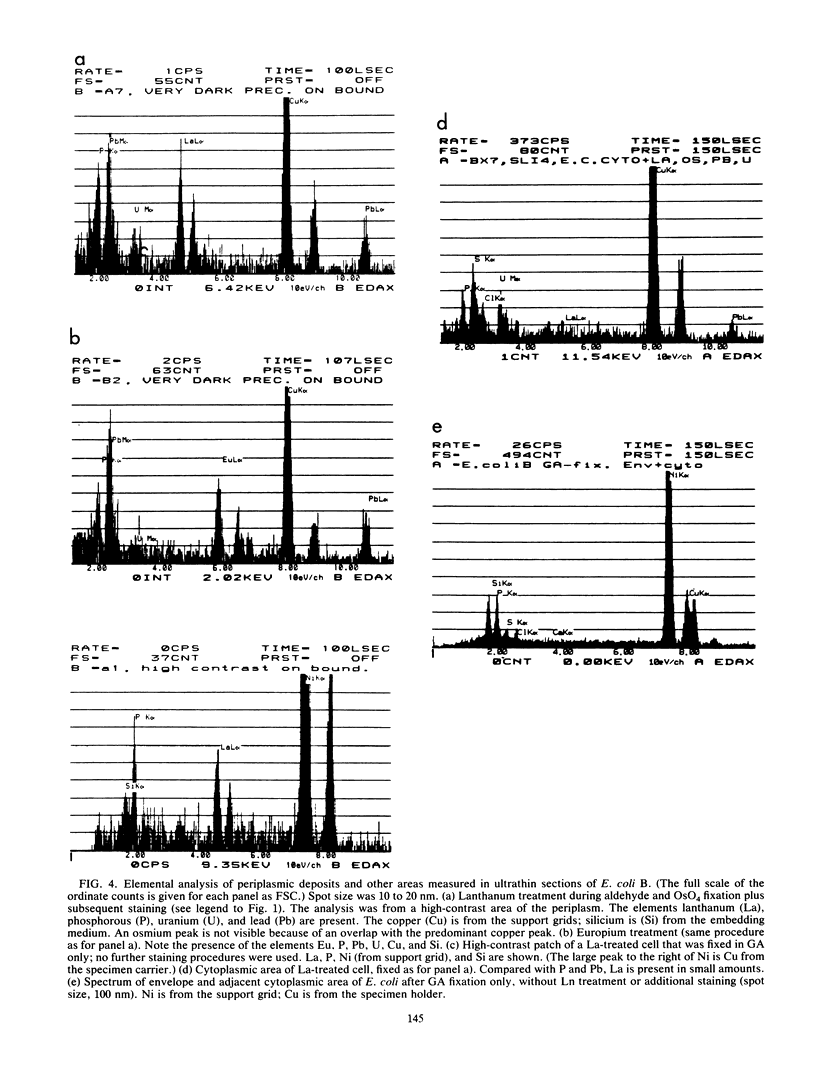
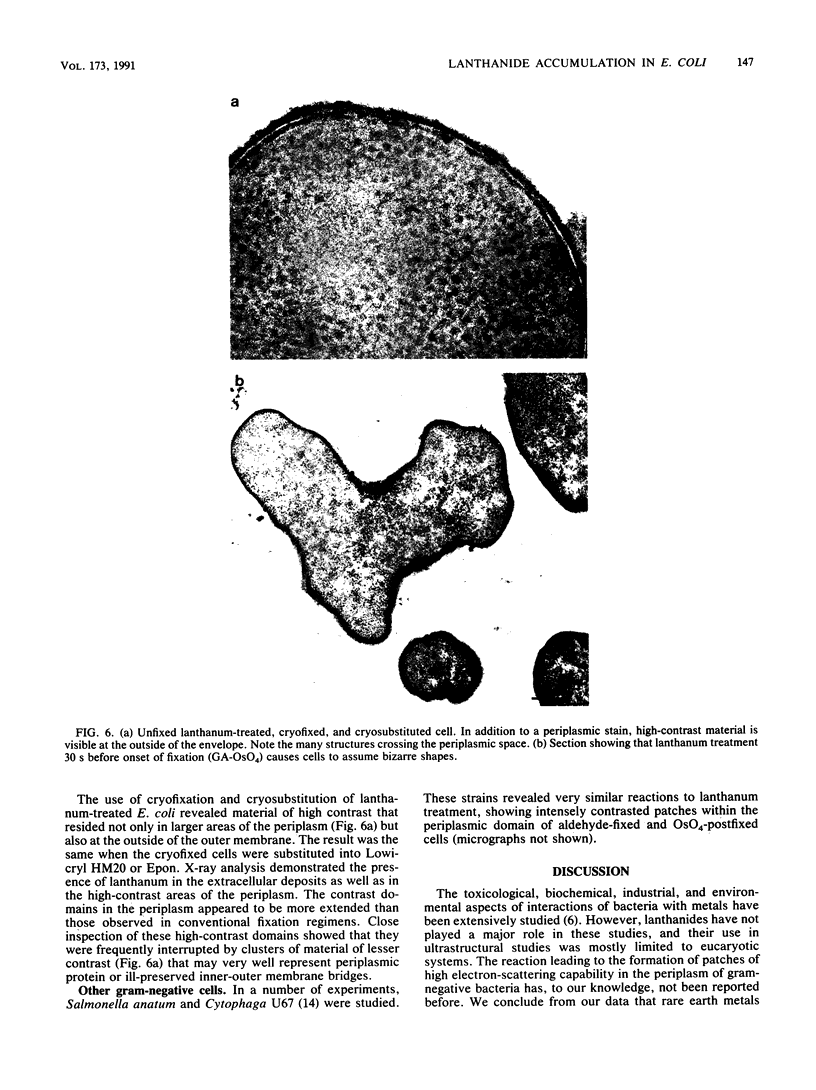
Treatment of growing Escherichia coli B with lanthanide ions [lanthanum(III), terbium(III), and europium(III)] and subsequent aldehyde-OsO4 fixation caused areas of high contrast to appear within the periplasm (the space between inner and outer membrane of the cell envelope). X-ray microanalysis of ultrathin sections of Epon-embedded or acrylic resin-embedded cells revealed the presence of the lanthanide and of phosphorus in the areas, whose contrast greatly exceeded that of other stained structures. Comparatively small amounts of the lanthanide were also present in the outer membrane and in the cytoplasm. The distribution of the periplasmic areas of high contrast was found to be random and not clustered at areas of current or future septum formation. Irregular cell shapes were observed after lanthanide treatment before onset of fixation. In contrast to glutaraldehyde-OsO4 fixation, glutaraldehyde used as the sole fixer caused a scattered distribution of the lanthanide. Cryofixation (slam-freezing) and freeze substitution revealed a lanthanum stain at both the periplasm and the outer part of the outer membrane. Deenergization of the cell membrane by either phage T4 or carbonyl cyanide m-chlorophenylhydrazone abolished the metal accumulation. Furthermore, addition of excess calcium, administered together with the lanthanide solution, diminished the quantity and size of areas of high contrast. Cells grown in media of high NaCl concentration revealed strongly stained areas of periplasmic precipitates, whereas cells grown under low-salt conditions showed very few high-contrast patches in the periplasm. Terbium treatment (during fixation) enhanced the visibility of the sites of inner-outer membrane contact (the membrane adhesion sites) in plasmolized cells, possibly as the result of an accumulation of the metal at the adhesion domains. The data suggest a rapid interaction of the lanthanides with components of the cell envelope, the periplasm, and the energized inner membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F. The morphology and osmotic properties of bacteriophage systems. Cold Spring Harb Symp Quant Biol. 1953;18:197–203. doi: 10.1101/sqb.1953.018.01.030. [DOI] [PubMed] [Google Scholar]
- Agar N. S., Suzuki T., Roberts J., Evans J. V. Effect of anaemia on red cell metabolism in cattle. Comp Biochem Physiol B. 1983;75(3):445–449. doi: 10.1016/0305-0491(83)90356-5. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Bayer M. H. Fast responses of bacterial membranes to virus adsorption: a fluorescence study. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5618–5622. doi: 10.1073/pnas.78.9.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Koval S. F. Binding of metals to cell envelopes of Escherichia coli K-12. Appl Environ Microbiol. 1981 Aug;42(2):325–335. doi: 10.1128/aem.42.2.325-335.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980 Feb;141(2):876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada R. G. Terbium binding to neoplastic GH3 pituitary cells. Biochem Biophys Res Commun. 1983 Feb 28;111(1):135–142. doi: 10.1016/s0006-291x(83)80127-2. [DOI] [PubMed] [Google Scholar]
- Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972 Dec;36(4):478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayne M. S., Cohn M. Enhancement of Tb(III) and Eu(III) fluorescence in complexes with Escherichia coli tRNA. Biochemistry. 1974 Sep 24;13(20):4159–4165. doi: 10.1021/bi00717a014. [DOI] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B., Letellier L. Membrane potential changes during the first steps of coliphage infection. Proc Natl Acad Sci U S A. 1981 Jan;78(1):215–219. doi: 10.1073/pnas.78.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalister T. J., Macdonald B., Rothfield L. I. The periseptal annulus: An organelle associated with cell division in Gram-negative bacteria. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1372–1376. doi: 10.1073/pnas.80.5.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Karnovsky M. J. Ultrastructural localization of several phosphatases with cerium. J Histochem Cytochem. 1983 Oct;31(10):1197–1208. doi: 10.1177/31.10.6309949. [DOI] [PubMed] [Google Scholar]
- Shaklai M., Tavassoli M. Preferential localization of lanthanum to nuclear-pore complexes. J Ultrastruct Res. 1982 Nov;81(2):139–144. doi: 10.1016/s0022-5320(82)90069-7. [DOI] [PubMed] [Google Scholar]
- Soini E., Hemmilä I. Fluoroimmunoassay: present status and key problems. Clin Chem. 1979 Mar;25(3):353–361. [PubMed] [Google Scholar]