Abstract
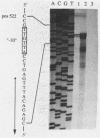
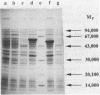
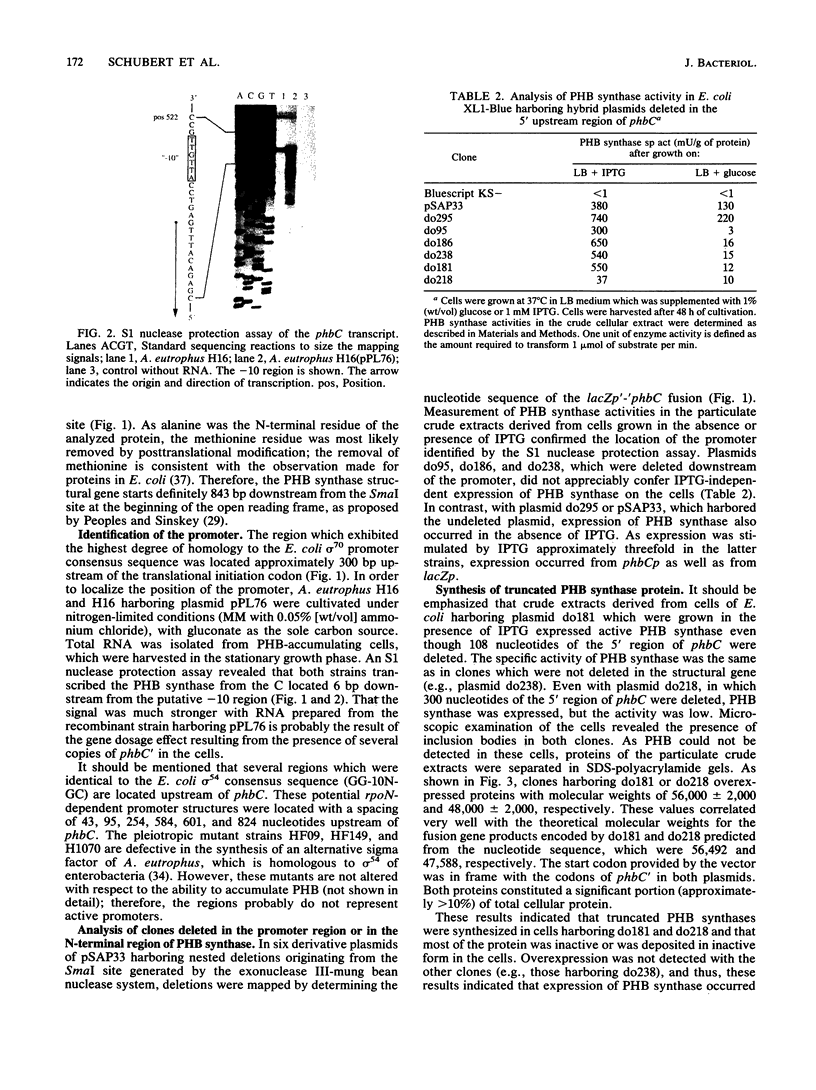
Molecular methods have been applied to analyze the expression of the Alcaligenes eutrophus poly(3-hydroxybutyrate) (PHB) synthase gene (phbC). The translational initiation codon was identified by analysis of the amino acid sequence of a PHB synthase-beta-galactosidase fusion protein. This protein was purified to almost gel electrophoretic homogeneity by chromatography on DEAE-Sephacel and on aminophenyl-beta-D-thiogalactopyranoside-Sepharose from cells of A. eutrophus which harbored a phbC'-'lacZ fusion gene. A sequence (TTGACA-18N-AACAAT), exhibiting striking homology to the Escherichia coli sigma 70 promoter consensus sequence, was identified approximately 310 bp 5' upstream from the translation initiation codon. An S1 nuclease protection assay mapped the transcription start point of phbC 6 bp downstream from this promoter. The location of the promoter was confirmed by analyzing the expression of active PHB synthase in clones of E. coli harboring 5' upstream deletions of phbC ligated to the promoter of the lacZ gene (lacZp) in a Bluescript vector. Plasmids do181 and do218, which were deleted for the first 108 or 300 bp of the phbC structural gene, respectively, conferred the ability to synthesize large amounts of different truncated PHB synthase proteins to the cells. These proteins contributed to approximately 10% of the total cellular protein as estimated from sodium dodecyl sulfate-polyacrylamide gels. The modified PHB synthase encoded by plasmid do181 was still active. Clones in which the lacZp-'phbC fusion harbored the complete phbC structural gene plus the phbC ribosome binding site did not overexpress PHB synthase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M., Claverie-Martín F., Díaz-Torres M. R., Kushner S. R. Transcript mapping using [35S]DNA probes, trichloroacetate solvent and dideoxy sequencing ladders: a rapid method for identification of transcriptional start points. Gene. 1988 May 15;65(1):101–110. doi: 10.1016/0378-1119(88)90421-0. [DOI] [PubMed] [Google Scholar]
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Hogrefe C., Schlegel H. G. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol. 1981 Jul;147(1):198–205. doi: 10.1128/jb.147.1.198-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fründ C., Priefert H., Steinbüchel A., Schlegel H. G. Biochemical and genetic analyses of acetoin catabolism in Alcaligenes eutrophus. J Bacteriol. 1989 Dec;171(12):6539–6548. doi: 10.1128/jb.171.12.6539-6548.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T., Yoshimoto A., Matsumoto M., Hosokawa S., Saito T. Enzymatic synthesis of poly-beta-hydroxybutyrate in Zoogloea ramigera. Arch Microbiol. 1976 Nov 2;110(23):149–156. doi: 10.1007/BF00690222. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hogrefe C., Römermann D., Friedrich B. Alcaligenes eutrophus hydrogenase genes (Hox). J Bacteriol. 1984 Apr;158(1):43–48. doi: 10.1128/jb.158.1.43-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrossek D., Krüger N., Steinbüchel A. Characterization of alcohol dehydrogenase genes of derepressible wild-type Alcaligenes eutrophus H16 and constitutive mutants. J Bacteriol. 1990 Sep;172(9):4844–4851. doi: 10.1128/jb.172.9.4844-4851.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrossek D., Steinbüchel A., Schlegel H. G. Alcohol dehydrogenase gene from Alcaligenes eutrophus: subcloning, heterologous expression in Escherichia coli, sequencing, and location of Tn5 insertions. J Bacteriol. 1988 Nov;170(11):5248–5256. doi: 10.1128/jb.170.11.5248-5256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Saito T., Tomita K. Purification and properties of beta-ketothiolase from Zoogloea ramigera. Arch Microbiol. 1978 Jan 23;116(1):21–27. doi: 10.1007/BF00408729. [DOI] [PubMed] [Google Scholar]
- Peoples O. P., Sinskey A. J. Poly-beta-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC). J Biol Chem. 1989 Sep 15;264(26):15298–15303. [PubMed] [Google Scholar]
- Peoples O. P., Sinskey A. J. Poly-beta-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding beta-ketothiolase and acetoacetyl-CoA reductase. J Biol Chem. 1989 Sep 15;264(26):15293–15297. [PubMed] [Google Scholar]
- Pool R. In Search of the Plastic Potato: Scientists in the emerging field of biopolymer engineering are aiming to produce bacteria and, eventually, food crops that are genetically tailored to yield a whole new breed of plastics. Science. 1989 Sep 15;245(4923):1187–1189. doi: 10.1126/science.245.4923.1187. [DOI] [PubMed] [Google Scholar]
- Rodriguez H., Kohr W. J., Harkins R. N. Design and operation of a completely automated Beckman microsequencer. Anal Biochem. 1984 Aug 1;140(2):538–547. doi: 10.1016/0003-2697(84)90205-7. [DOI] [PubMed] [Google Scholar]
- Römermann D., Warrelmann J., Bender R. A., Friedrich B. An rpoN-like gene of Alcaligenes eutrophus and Pseudomonas facilis controls expression of diverse metabolic pathways, including hydrogen oxidation. J Bacteriol. 1989 Feb;171(2):1093–1099. doi: 10.1128/jb.171.2.1093-1099.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarimo S. S., Pine M. J. Taxonomic comparison of the amino termini of microbial cell proteins. J Bacteriol. 1969 May;98(2):368–374. doi: 10.1128/jb.98.2.368-374.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. Mutants of Alcaligenes eutrophus defective in autotrophic metabolism. Arch Microbiol. 1978 May 30;117(2):123–129. doi: 10.1007/BF00402299. [DOI] [PubMed] [Google Scholar]
- Schubert P., Steinbüchel A., Schlegel H. G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988 Dec;170(12):5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S. C., Voige W. H., Dennis D. E. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1988 Oct;170(10):4431–4436. doi: 10.1128/jb.170.10.4431-4436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm A., Steinbüchel A. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl Environ Microbiol. 1990 Nov;56(11):3360–3367. doi: 10.1128/aem.56.11.3360-3367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Betcke A., Warnecke U., Böcker C., Zaborosch C., Friedrich B. Cloning and nucleotide sequences of the genes for the subunits of NAD-reducing hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1990 Jun;172(6):2920–2929. doi: 10.1128/jb.172.6.2920-2929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann A. One-step purification of hybrid proteins which have beta-galactosidase activity. Gene. 1984 Jul-Aug;29(1-2):27–31. doi: 10.1016/0378-1119(84)90162-8. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]