Abstract
1 The efflux, from heart ventricular strips of Rana pipiens, of sodium (22Na) and calcium (45Ca) was measured simultaneously.
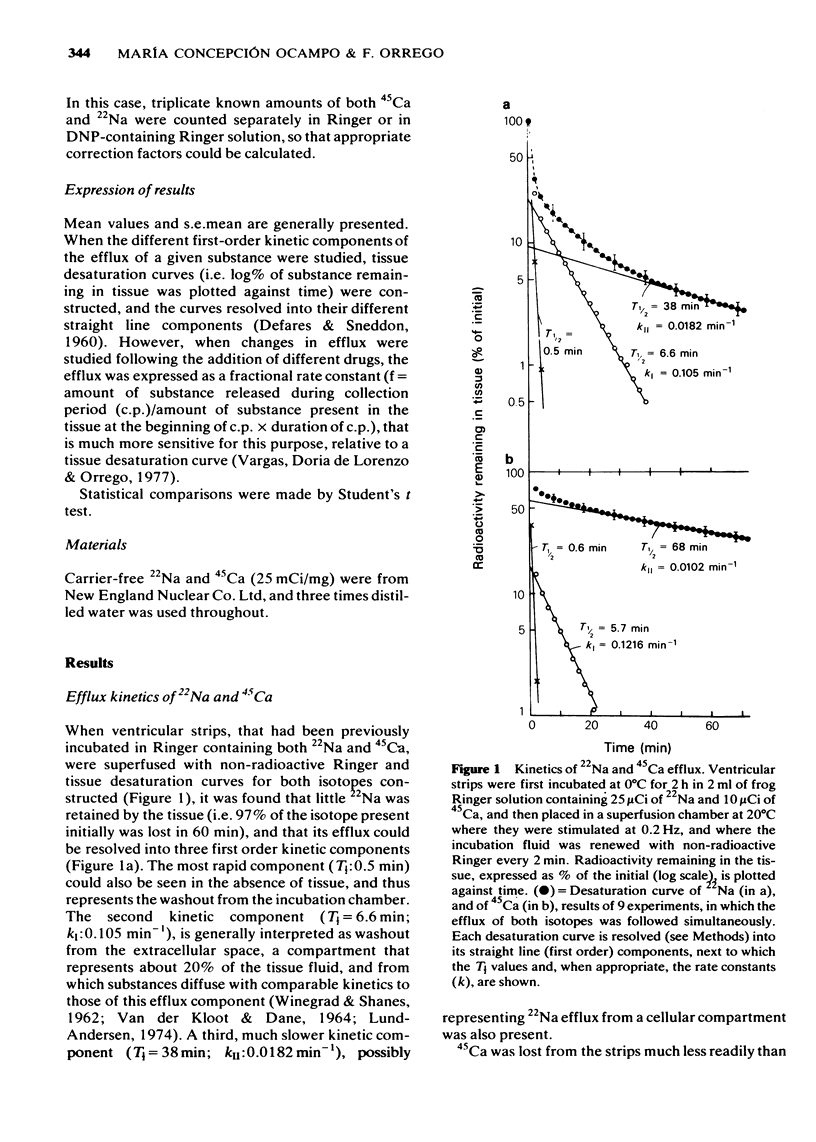
2 22Na efflux could be resolved into two first order kinetic components: kI = 0.105 min-1, thought to represent efflux from the extracellular space, and kII = 0.0182 min-1 representing efflux from the cells.
3 45Ca efflux was also resolved into an extracellular component, kI = 0.1216 min-1; and an intracellular one, kII = 0.0102 min-1. 45Ca kII was greatly increased by 2,4-dinitrophenol (DNP), but unchanged by caffeine. This suggests that it represents a mitochondrial calcium compartment.
4 22Na kII was not changed by DNP. This indicates that, at the time of DNP addition, 22Na was passively bound to undefined intracellular components.
5 Ouabain (10-6 M) decreased 45Ca efflux (kII) initially but at later periods slightly increased it. The former effect is thought to be due to an action at the plasma membrane level, while the latter probably represents an increased exchangeability of mitochondrial calcium. The same effects were always found when ouabain was applied at different times of strip superfusion.
6 Ouabain (0.25 to 4 μM) did not decrease the kII of 22Na efflux. Kinetic reasons are presented which indicate that, in this preparation, the activity of the sodium pump may be too fast to be measured by means of 22Na efflux, therefore these findings do not necessarily mean that ouabain does not inhibit active sodium transport.
7 The time course of the inotropic effect of ouabain was also studied in ventricular strips of Rana pipiens heart that were stimulated at 0.2 Hz with biphasic, 2 ms pulses of supramaximal intensity, and incubated in Ringer solution containing 1.1 mM calcium, or in `calcium-free' Ringer (residual calcium: 5.2 μM), or in `calcium-free' Ringer with 0.1 mM of the calcium chelator ethyleneglycol bis (β-aminoethylether) N,N′-tetraacetic acid (EGTA).
8 In Ringer, the inotropic effect of ouabain was already observed at 5-10 s after steroid addition, even with the lowest concentration tested (0.25 μM), while signs of toxicity appeared only after 15 min in 4 μM ouabain, the highest concentration used.
9 When the strips were incubated in `calcium-free' Ringer solution, force of contraction decayed to 1-2% of that in 1.1 mM calcium. Addition of 4 μM ouabain to these hypodynamic strips led to a progressive increase in contractile force of up to 300%, that started after a 50 s latency period. No signs of toxicity were observed.
10 Incubation of the strips in EGTA-Ringer also reduced contractile force to about 2% of that in Ringer, and 4 μM ouabain also increased force of contraction by approximately the same amount as seen in `calcium-free' Ringer, but the effect began after a 10 min latency period. The concentration of calcium ion (Ca2+) in the extracellular space of strips incubated in EGTA-Ringer, was approximately 800 fold lower than in Ringer, and 60 fold lower than in `calcium-free' Ringer solution.
11 Caffeine (20 mM) induced, in strips previously incubated for 1 h in 4.4 mM calcium Ringer solution plus 10-6 M ouabain, a marked initial contracture, that relaxed spontaneously, and was followed by slow waves of contracture. This was not observed if the strips were incubated, prior to caffeine, in 4.4 mM calcium Ringer without ouabain, or in 1.1 mM calcium Ringer solution that contained 10-6 M ouabain.
12 Based on these findings, a hypothesis that can explain the inotropic effect of cardioactive steroids is presented.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Bennett R. T., Olgaard M. K., Brody T. M. Cardiac Na+, K+-adenosine triphosphatase inhibition by ouabain and myocardial sodium: a computer simulation. J Pharmacol Exp Ther. 1976 Nov;199(2):287–297. [PubMed] [Google Scholar]
- Akera T., Brody T. M. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev. 1977 Sep;29(3):187–220. [PubMed] [Google Scholar]
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr Ionic currents in cardiac muscle: a framework for glycoside action. Fed Proc. 1977 Aug;36(9):2209–2213. [PubMed] [Google Scholar]
- Bentfeld M., Lüllmann H., Peters T., Proppe D. Interdependence of ion transport and the action of quabain in heart muscle. Br J Pharmacol. 1977 Sep;61(1):19–27. doi: 10.1111/j.1476-5381.1977.tb09735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Miller D. J. The effects of caffeine on the contraction of the frog heart. J Physiol. 1974 Nov;242(3):589–613. doi: 10.1113/jphysiol.1974.sp010725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke W. J., Robinson J. D. Factors influencing calcium movements in rat brain slices. Am J Physiol. 1971 Jul;221(1):218–225. doi: 10.1152/ajplegacy.1971.221.1.218. [DOI] [PubMed] [Google Scholar]
- Dale A. S. The staircase phenomenon in ventricular muscle. J Physiol. 1932 May 30;75(1):1–16. doi: 10.1113/jphysiol.1932.sp002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci. 1978 Apr 28;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino S., Igarashi T., Hoshi K. Ouabain potentiation of Ca release from fragmented cardiac sarcoplasmic reticulum. Jpn J Pharmacol. 1979 Dec;29(6):839–845. doi: 10.1254/jjp.29.839. [DOI] [PubMed] [Google Scholar]
- Ghysel-Burton J., Godfraind T. Stimulation and inhibition of the sodium pump by cardioactive steroids in relation to their binding sites and their inotropic effect on guinea-pig isolated atria. Br J Pharmacol. 1979 Jun;66(2):175–184. doi: 10.1111/j.1476-5381.1979.tb13662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAUS W., KUSCHINSKY G. [On the effect of digitoxigenin on the cellular calcium exchange in heart muscle tissue]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1962;244:237–253. [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T. Physiological significance of Ca uptake by mitochondria in the heart in comparison with that by cardiac sarcoplasmic reticulum. J Biochem. 1976 Nov;80(5):1129–1147. doi: 10.1093/oxfordjournals.jbchem.a131369. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Relationship between myocardial contractility and the effects of digitalis on ionic exchange. Fed Proc. 1977 Aug;36(9):2231–2234. [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Klaus W. The subcellular basis for the mechanism of inotropic action of cardiac glycosides. Pharmacol Rev. 1971 Sep;23(3):193–261. [PubMed] [Google Scholar]
- Lund-Andersen H. Extracellular and intracellular distribution of inulin in rat brain cortex slices. Brain Res. 1974 Jan 11;65(2):239–254. doi: 10.1016/0006-8993(74)90036-5. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Narita K. Caffeine contracture in frog cardiac muscle after exposure to high concentrations of calcium. Jpn J Physiol. 1980;30(1):137–141. doi: 10.2170/jjphysiol.30.137. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Nawrath H., Trautwein W. Membrane currents and tension in cat ventricular muscle treated with cardiac glycosides. Circ Res. 1975 Nov;37(5):674–682. doi: 10.1161/01.res.37.5.674. [DOI] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. The staircase phenomenon and the action of calcium on the heart. J Physiol. 1956 Dec 28;134(3):569–583. doi: 10.1113/jphysiol.1956.sp005666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G. An effect of ouabain on the superficially-located stores of calcium in cardiac muscle cells. J Mol Cell Cardiol. 1973 Feb;5(1):101–110. doi: 10.1016/0022-2828(73)90039-4. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., NONOMURA Y. The influence of ouabain on the relation between membrane potential and tension in frog heart muscle. J Pharmacol Exp Ther. 1963 Jul;141:1–5. [PubMed] [Google Scholar]
- Okita G. T. Dissociation of Na+,K+-ATPase inhibition from digitalis inotropy. Fed Proc. 1977 Aug;36(9):2225–2230. [PubMed] [Google Scholar]
- Orrego F., Jankelevich J., Ceruti L., Ferrera E. Differential effects of electrical stimulation on release of 3H-noradrenaline and 14C-alpha-aminoisobutyrate from brain slices. Nature. 1974 Sep 6;251(5470):55–56. doi: 10.1038/251055a0. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Carafoli E. A study of the intracellular transport of calcium in rat heart. J Cell Physiol. 1968 Aug;72(1):29–37. doi: 10.1002/jcp.1040720106. [DOI] [PubMed] [Google Scholar]
- Pietrzyk C., Heinz E. The sequestration of Na+, K+ and Cl- in the cellular nucleus and its energetic consequences for the gradient hypothesis of amino acid transport in Ehrlich cells. Biochim Biophys Acta. 1974 Jun 29;352(3):397–411. doi: 10.1016/0005-2736(74)90231-4. [DOI] [PubMed] [Google Scholar]
- SLEATOR W., Jr, FURCHGOTT R. F., DE GUBAREFF T., KRESPI V. ACTION POTENTIALS OF GUINEA PIG ATRIA UNDER CONDITIONS WHICH ALTER CONTRACTION. Am J Physiol. 1964 Feb;206:270–282. doi: 10.1152/ajplegacy.1964.206.2.270. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Graziotti P. Mechanisms for intracellular calcium regulation in heart. I. Stopped-flow measurements of Ca++ uptake by cardiac mitochondria. J Gen Physiol. 1973 Dec;62(6):756–772. doi: 10.1085/jgp.62.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert G., Langendorf H., Hannover R., Nitz-Litzow D., Pressman B. C., Moore C. Untersuchungen zur Rolle des Natrium-Stoffwechels im Zellkern der Rattenleber. Hoppe Seylers Z Physiol Chem. 1965;343(1):101–115. [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative ultrastructural study with special reference to frog and chicken hearts. Z Zellforsch Mikrosk Anat. 1969;98(3):437–468. [PubMed] [Google Scholar]
- Staley N. A., Benson E. S. The ultrastructure of frog ventricular cardiac muscle and its relationship to mechanism of excitation-contraction coupling. J Cell Biol. 1968 Jul;38(1):99–114. doi: 10.1083/jcb.38.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUTTLE R. S., WITT P. N., FARAH A. The influence of ouabain on intracellular sodium and potassium concentrations in the rabbit myocardium. J Pharmacol Exp Ther. 1961 Sep;133:281–287. [PubMed] [Google Scholar]
- VANDERKLOTT W. G., DANE B. THE EFFLUX OF SUBSTANCES FROM FROG VENTRICLES TO SUCROSE AND TO RINGER'S SOLUTIONS. J Gen Physiol. 1964 Nov;48:199–224. doi: 10.1085/jgp.48.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas O., Del Carmen Doria de Lorenzo M., Orrego F. Effect of elevated extracellular potassium on the release of labelled noradrenaline, glutamate, glycine, beta-alanine and other amino acids from rat brain cortex slices. Neuroscience. 1977;2(3):383–390. doi: 10.1016/0306-4522(77)90004-5. [DOI] [PubMed] [Google Scholar]
- Vercesi A., Reynafarje B., Lehninger A. L. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J Biol Chem. 1978 Sep 25;253(18):6379–6385. [PubMed] [Google Scholar]
- WINEGRAD S., SHANES A. M. Calcium flux and contractility in guinea pig atria. J Gen Physiol. 1962 Jan;45:371–394. doi: 10.1085/jgp.45.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J Gen Physiol. 1968 Nov;52(5):760–772. doi: 10.1085/jgp.52.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., Schwartz A. Effects of ouabain on calcium-45 flux in guinea pig cardiac tissue. J Mol Cell Cardiol. 1978 Feb;10(2):137–144. doi: 10.1016/0022-2828(78)90038-x. [DOI] [PubMed] [Google Scholar]


