Abstract
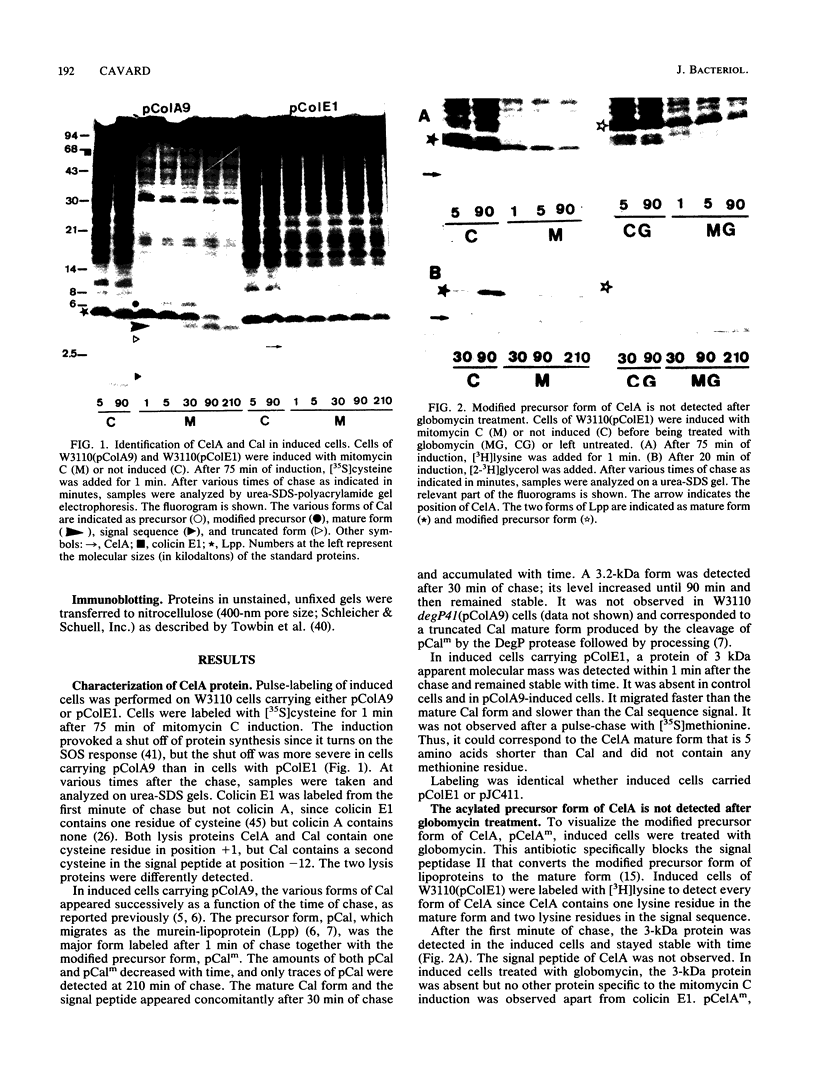
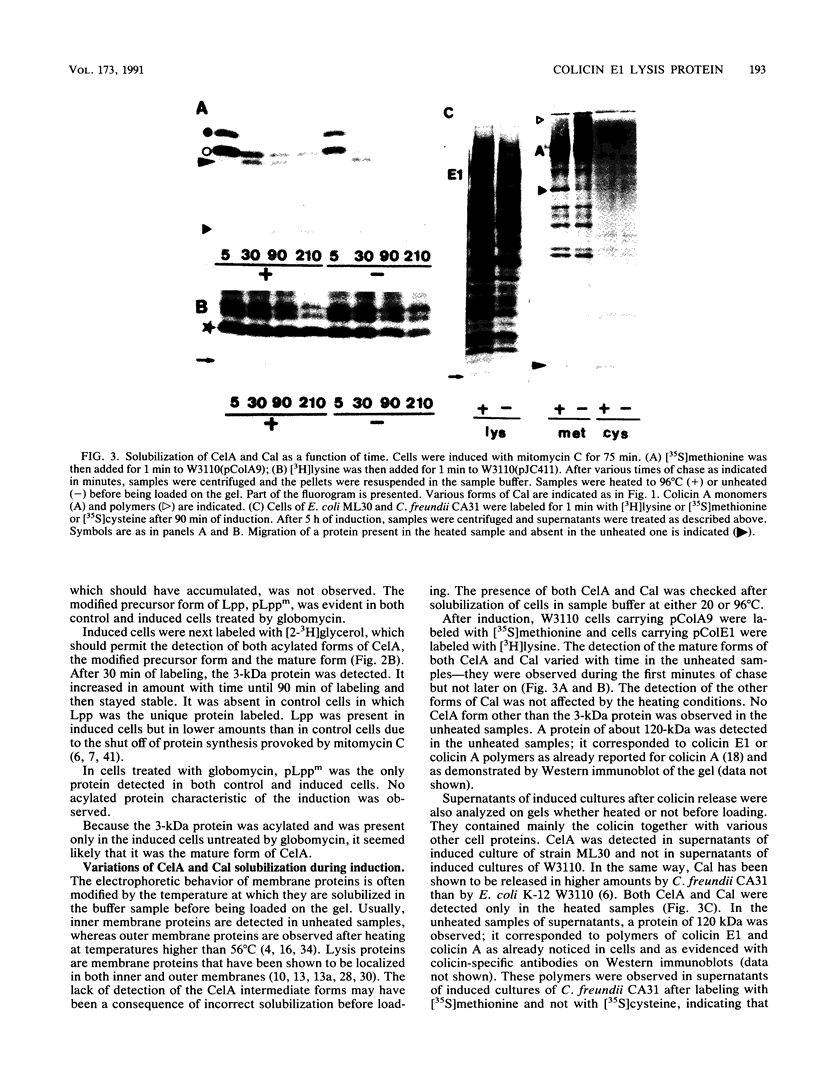
The colicin E1 lysis protein, CelA, was identified as a 3-kDa protein in induced cells of Escherichia coli K-12 carrying pColE1 by pulse-chase labeling with either [35S]cysteine or [3H]lysine. This 3-kDa protein was acylated, as shown by [2-3H]glycerol labeling, and seemed to correspond to the mature CelA protein. The rate of modification and processing of CelA was different from that observed for Cal, the colicin A lysis protein. In contrast to Cal, no intermediate form was detected for CelA, no signal peptide accumulated, and no modified precursor form was observed after globomycin treatment. Thus, the rate of synthesis would not be specific to lysis proteins. Solubilization in sodium dodecyl sulfate of the mature forms of both CelA and Cal varied similarly at the time of colicin release, indicating a change in lysis protein structure. This particular property would play a role in the mechanism of colicin export. The accumulation of the signal peptide seems to be a factor determining the toxicity of the lysis proteins since CelA provoked less cell damage than Cal. Quasi-lysis and killing due to CelA were higher in degP mutants than in wild-type cells. They were minimal in pldA mutants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri M., Suit J. L., Fan M. L., Luria S. E. Expression of the cloned ColE1 kil gene in normal and Kilr Escherichia coli. J Bacteriol. 1986 Nov;168(2):648–654. doi: 10.1128/jb.168.2.648-654.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono R. Probable detection of kil peptide derived from colicin E1 plasmid in the envelope fraction of Escherichia coli HB101 carrying pEAP31. Biochem J. 1988 Oct 1;255(1):365–368. [PMC free article] [PubMed] [Google Scholar]
- Baty D., Lloubès R., Geli V., Lazdunski C., Howard S. P. Extracellular release of colicin A is non-specific. EMBO J. 1987 Aug;6(8):2463–2468. doi: 10.1002/j.1460-2075.1987.tb02526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla J. M., Lazdunski C., Pagès J. M. The assembly of the major outer membrane protein OmpF of Escherichia coli depends on lipid synthesis. EMBO J. 1988 Nov;7(11):3595–3599. doi: 10.1002/j.1460-2075.1988.tb03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Howard S. P., Lazdunski C. Functioning of the colicin A lysis protein is affected by Triton X-100, divalent cations and EDTA. J Gen Microbiol. 1989 Jun;135(6):1715–1726. doi: 10.1099/00221287-135-6-1715. [DOI] [PubMed] [Google Scholar]
- Cavard D., Howard S. P., Lloubes R., Lazdunski C. High-level expression of the colicin A lysis protein. Mol Gen Genet. 1989 Jun;217(2-3):511–519. doi: 10.1007/BF02464925. [DOI] [PubMed] [Google Scholar]
- Cavard D., Lazdunski C., Howard S. P. The acylated precursor form of the colicin A lysis protein is a natural substrate of the DegP protease. J Bacteriol. 1989 Nov;171(11):6316–6322. doi: 10.1128/jb.171.11.6316-6322.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Lloubès R., Morlon J., Chartier M., Lazdunski C. Lysis protein encoded by plasmid ColA-CA31. Gene sequence and export. Mol Gen Genet. 1985;199(1):95–100. doi: 10.1007/BF00327516. [DOI] [PubMed] [Google Scholar]
- Chan P. T., Ohmori H., Tomizawa J., Lebowitz J. Nucleotide sequence and gene organization of ColE1 DNA. J Biol Chem. 1985 Jul 25;260(15):8925–8935. [PubMed] [Google Scholar]
- Cole S. T., Saint-Joanis B., Pugsley A. P. Molecular characterisation of the colicin E2 operon and identification of its products. Mol Gen Genet. 1985;198(3):465–472. doi: 10.1007/BF00332940. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Oudega B. Production and release of cloacin DF13 and related colicins. Curr Top Microbiol Immunol. 1986;125:183–205. doi: 10.1007/978-3-642-71251-7_11. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Nakazawa A. Cyclic AMP-dependent initiation and rho-dependent termination of colicin E1 gene transcription. J Biol Chem. 1983 Jun 10;258(11):7072–7078. [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. I. Localisation of the gene and identification and subcellular localisation of the gene H product. Mol Gen Genet. 1981;183(2):318–325. doi: 10.1007/BF00270635. [DOI] [PubMed] [Google Scholar]
- Howard S. P., Cavard D., Lazdunski C. Amino acid sequence and length requirements for assembly and function of the colicin A lysis protein. J Bacteriol. 1989 Jan;171(1):410–418. doi: 10.1128/jb.171.1.410-418.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai M., Takeuchi M., Shimizu K., Arai M. Mechanism of action of globomycin. J Antibiot (Tokyo) 1978 Nov;31(11):1203–1205. doi: 10.7164/antibiotics.31.1203. [DOI] [PubMed] [Google Scholar]
- Ito K. Identification of the secY (prlA) gene product involved in protein export in Escherichia coli. Mol Gen Genet. 1984;197(2):204–208. doi: 10.1007/BF00330964. [DOI] [PubMed] [Google Scholar]
- Jakes K. S., Model P. Mechanism of export of colicin E1 and colicin E3. J Bacteriol. 1979 Jun;138(3):770–778. doi: 10.1128/jb.138.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibiehler M., Lazdunski C. Conformation of colicin A: apparent difference between cytoplasmic and extracellular polypeptide chain. FEBS Lett. 1987 Jun 1;216(2):183–189. doi: 10.1016/0014-5793(87)80686-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Kato C., Kudo T., Horikoshi K. Excretion of the penicillinase of an alkalophilic Bacillus sp. through the Escherichia coli outer membrane is caused by insertional activation of the kil gene in plasmid pMB9. J Bacteriol. 1986 Jun;166(3):728–732. doi: 10.1128/jb.166.3.728-732.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P. C., Hefford M. A., Klein P. Structural relatedness of lysis proteins from colicinogenic plasmids and icosahedral coliphages. Mol Biol Evol. 1987 Sep;4(5):544–556. doi: 10.1093/oxfordjournals.molbev.a040460. [DOI] [PubMed] [Google Scholar]
- Lipinska B., Fayet O., Baird L., Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989 Mar;171(3):1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Zylicz M., Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990 Apr;172(4):1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloubes R., Baty D., Lazdunski C. The promoters of the genes for colicin production, release and immunity in the ColA plasmid: effects of convergent transcription and Lex A protein. Nucleic Acids Res. 1986 Mar 25;14(6):2621–2636. doi: 10.1093/nar/14.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., Clark D. M., Ras J., Verschoor E. J., Stegehuis F., de Graaf F. K., Oudega B. pCloDF13-encoded bacteriocin release proteins with shortened carboxyl-terminal segments are lipid modified and processed and function in release of cloacin DF13 and apparent host cell lysis. J Bacteriol. 1989 May;171(5):2673–2679. doi: 10.1128/jb.171.5.2673-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlon J., Chartier M., Bidaud M., Lazdunski C. The complete nucleotide sequence of the colicinogenic plasmid ColA. High extent of homology with ColE1. Mol Gen Genet. 1988 Feb;211(2):231–243. doi: 10.1007/BF00330599. [DOI] [PubMed] [Google Scholar]
- Morlon J., Lloubès R., Varenne S., Chartier M., Lazdunski C. Complete nucleotide sequence of the structural gene for colicin A, a gene translated at non-uniform rate. J Mol Biol. 1983 Oct 25;170(2):271–285. doi: 10.1016/s0022-2836(83)80148-x. [DOI] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Pattus F., Tucker A. D., Tsernoglou D. Structure of the membrane-pore-forming fragment of colicin A. Nature. 1989 Jan 5;337(6202):93–96. doi: 10.1038/337093a0. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Cole S. T. An unmodified form of the ColE2 lysis protein, an envelope lipoprotein, retains reduced ability to promote colicin E2 release and lysis of producing cells. J Gen Microbiol. 1987 Sep;133(9):2411–2420. doi: 10.1099/00221287-133-9-2411. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. A genetic approach to the study of mitomycin-induced lysis of Escherichia coli K-12 strains which produce colicin E2. Mol Gen Genet. 1983;190(3):366–372. doi: 10.1007/BF00331060. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. The immunity and lysis genes of ColN plasmid pCHAP4. Mol Gen Genet. 1988 Feb;211(2):335–341. doi: 10.1007/BF00330613. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Sabik J. F., Suit J. L., Luria S. E. cea-kil operon of the ColE1 plasmid. J Bacteriol. 1983 Mar;153(3):1479–1485. doi: 10.1128/jb.153.3.1479-1485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Johnson K., Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989 May;171(5):2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suit J. L., Fan M. L., Sabik J. F., Labarre R., Luria S. E. Alternative forms of lethality in mitomycin C-induced bacteria carrying ColE1 plasmids. Proc Natl Acad Sci U S A. 1983 Jan;80(2):579–583. doi: 10.1073/pnas.80.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba M., Masaki H., Ohta T. Primary structures of the ColE2-P9 and ColE3-CA38 lysis genes. J Biochem. 1986 Feb;99(2):591–596. doi: 10.1093/oxfordjournals.jbchem.a135515. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleh N. S., Johnson P. H. Structural and functional organization of the colicin E1 operon. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8389–8393. doi: 10.1073/pnas.82.24.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M., Tokunaga H., Hayashi S., Giam C. Z. Posttranslational modification and processing of membrane lipoproteins in bacteria. J Cell Biochem. 1983;22(3):161–171. doi: 10.1002/jcb.240220305. [DOI] [PubMed] [Google Scholar]
- Yamada M., Ebina Y., Miyata T., Nakazawa T., Nakazawa A. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein. Proc Natl Acad Sci U S A. 1982 May;79(9):2827–2831. doi: 10.1073/pnas.79.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. P., Faro A., Zubay G. Mitomycin-induced lethality of Escherichia coli cells containing the ColE1 Plasmid: involvement of the kil gene. J Bacteriol. 1985 Jul;163(1):174–179. doi: 10.1128/jb.163.1.174-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]