Abstract
1 The ability of cyproheptadine (Cph) to inhibit membrane translocation of calcium in smooth muscle was investigated by studying the drug's action on contraction, electrical activity and calcium influx in the guinea-pig taenia coli.
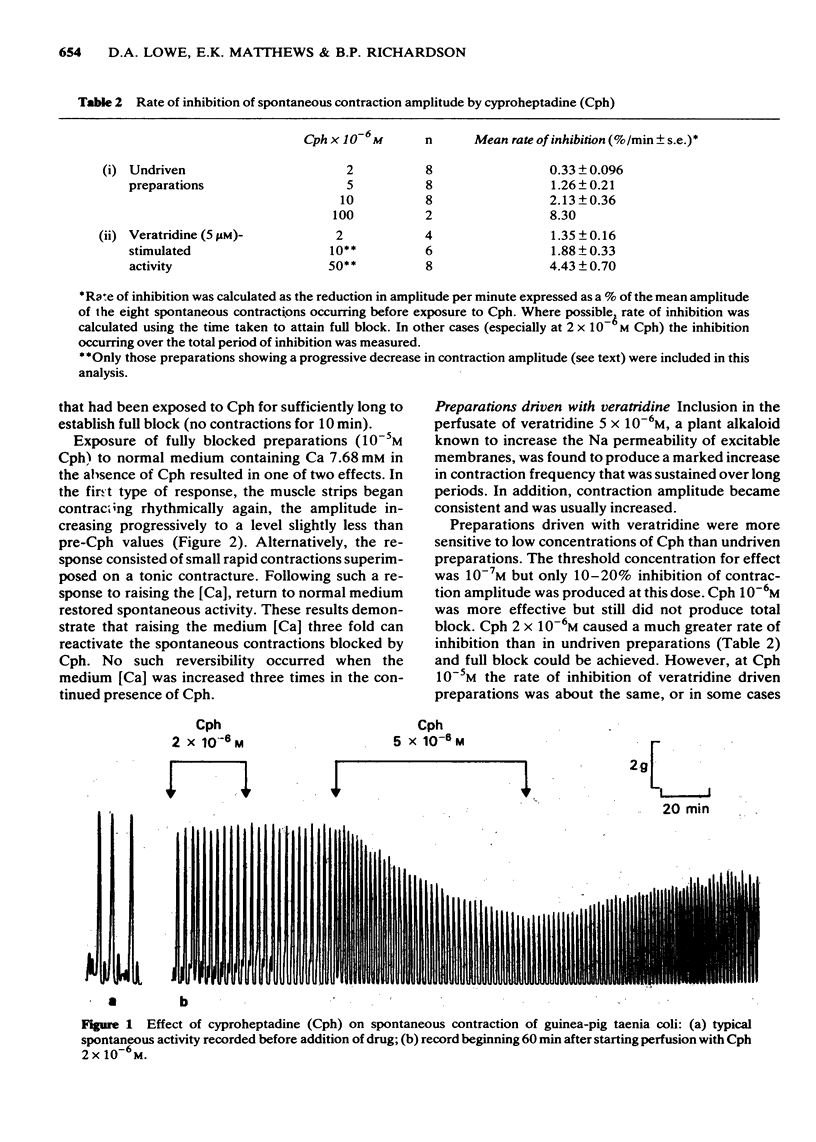
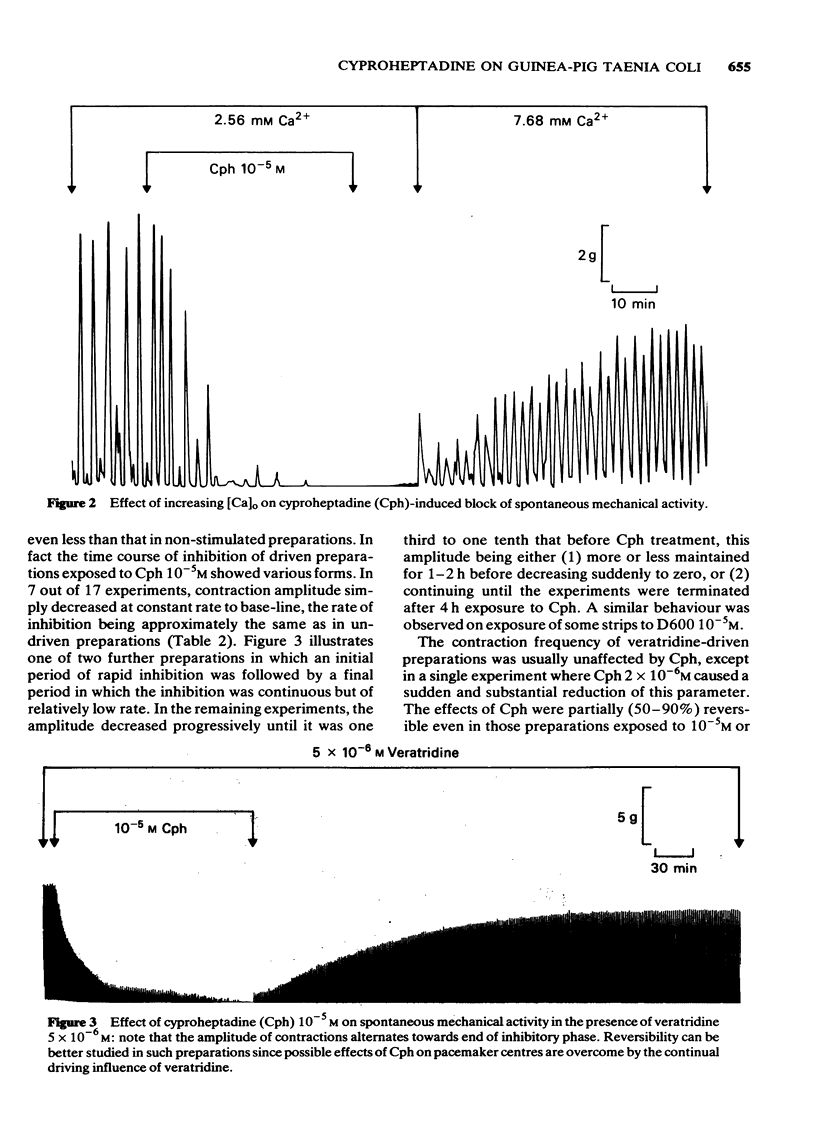
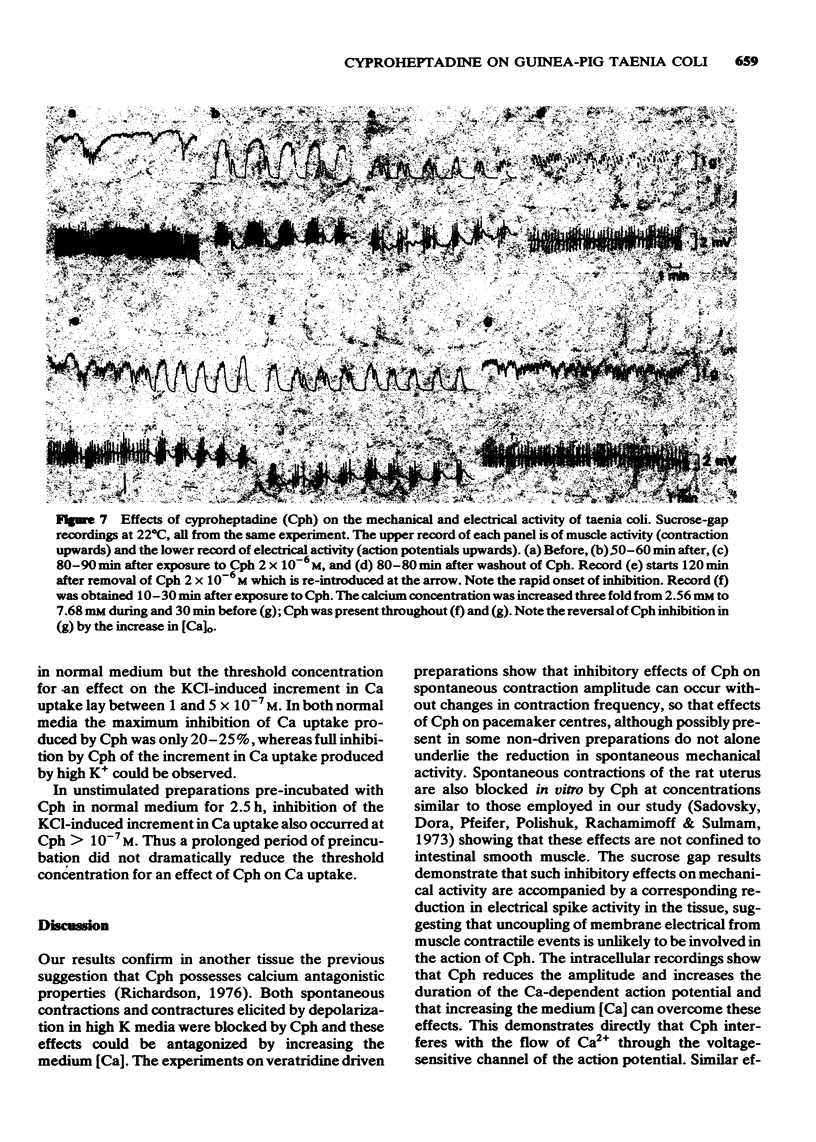
2 Cph ≥ 10-6M reduced the amplitude of normal spontaneous contractions and concurrently decreased the number of action potentials occurring with each slow-wave of depolarization (sucrose-gap recordings). These inhibitory effects of Cph were antagonized by increasing the medium [Ca] three fold to 7.68 mM.
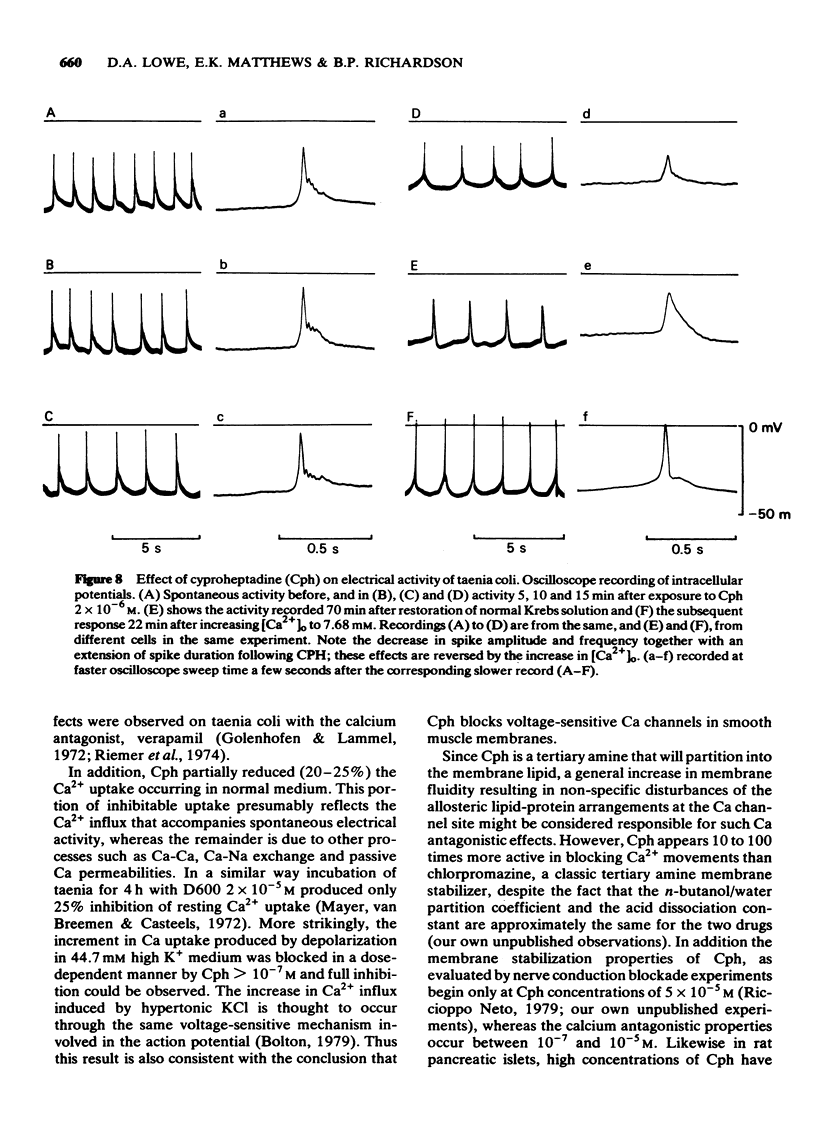
3 Intracellular recordings showed that Cph ≥ 2 × 10-6M decreased the amplitude and extended the duration of the action potential. These effects were only partially reversible in normal medium whereas large overshooting action potentials were again seen in 7.68 mM Ca medium.
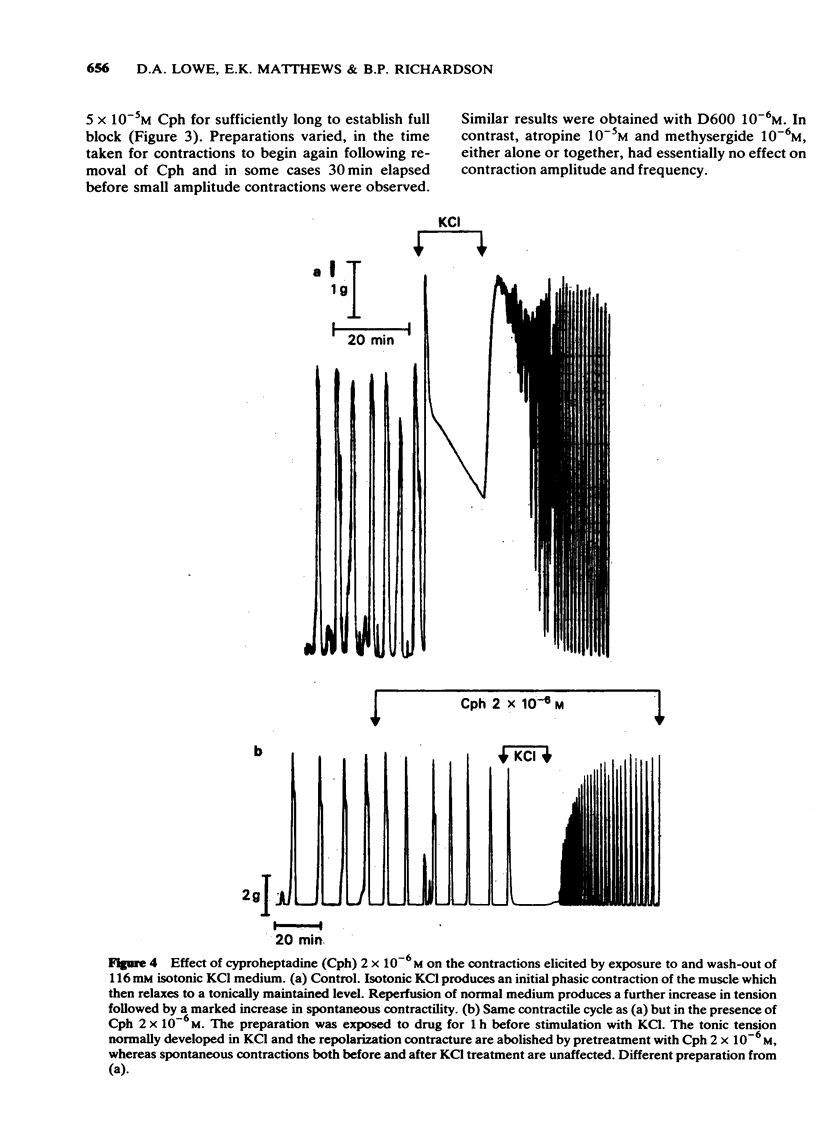
4 High frequency mechanical activity was produced by inclusion of veratridine 5 × 10-6M in the perfusate. Low concentrations of Cph (≥ 10-7M) reduced the amplitude of such contractions at a faster rate than they did normal spontaneous contractions.
5 At concentrations between 10-7 and 10-6M, Cph fully reduced the tonic component of contractions elicited in 112 mM isotonic KCl whilst having little or no effect on either (i) the initial phasic KCl contraction or (ii) the `repolarization contracture' normally produced on wash-out of the KCl or (iii) the spontaneous contractions before and after KCl treatment. In contrast, at Cph 2 × 10-6M, the repolarization contracture, as well as the isotonic KCl contraction, was totally blocked whereas spontaneous contractions were still unaffected. Progressively higher Cph concentrations inhibited all components of this contractile cycle.
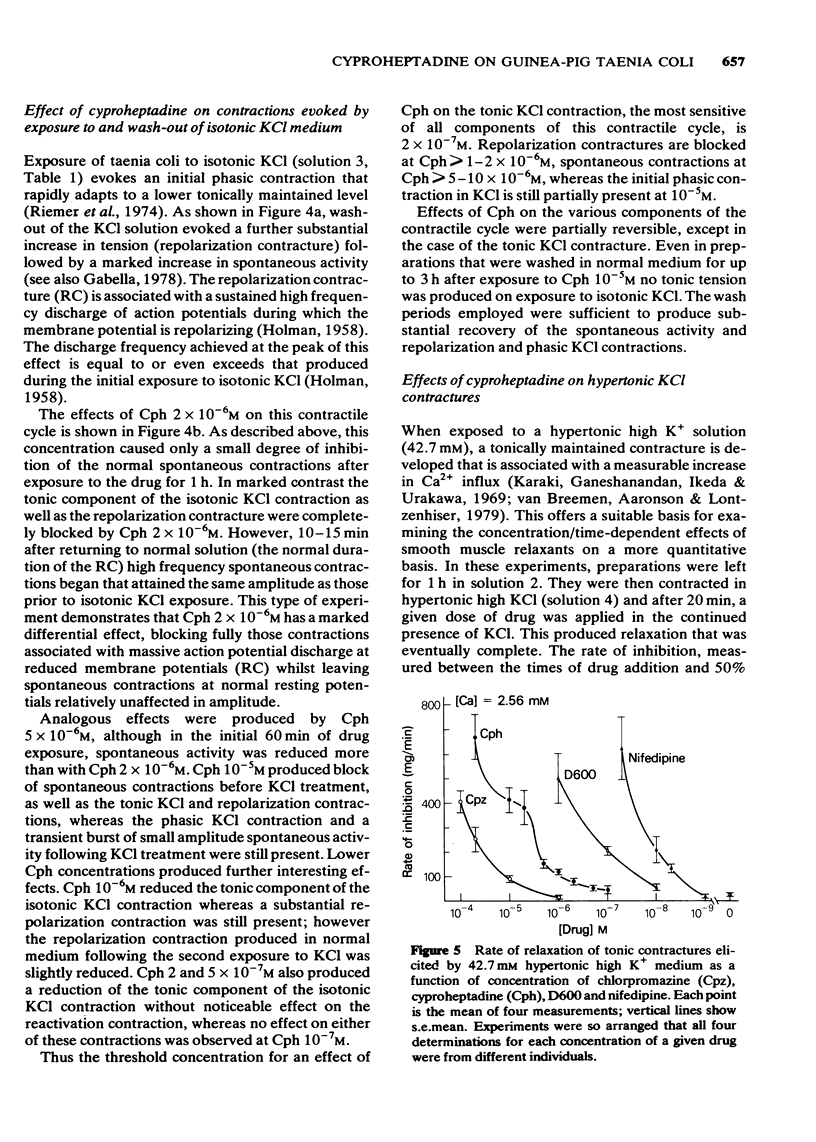
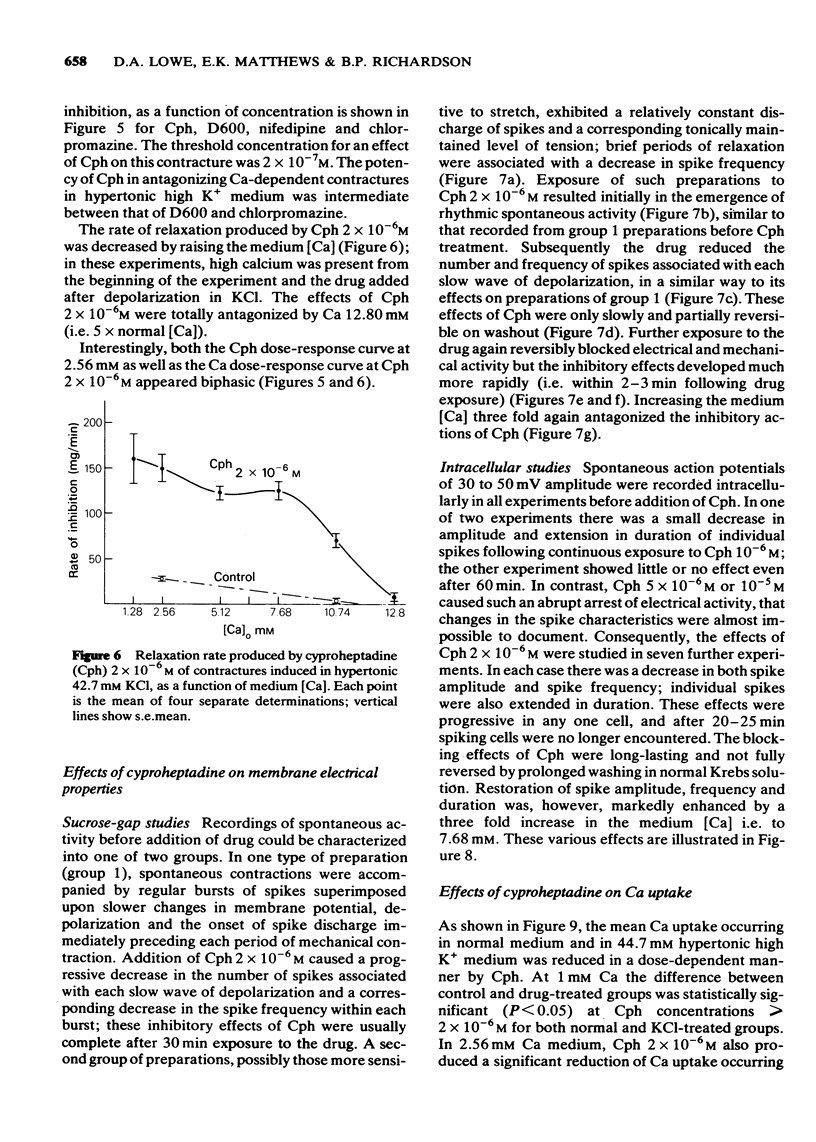
6 Dose-response curves for the rate of drug-induced relaxation of tonic contractures produced in hypertonic 42.7 mM high-potassium medium, showed the calcium antagonistic potency of Cph to be intermediate between that of chlorpromazine and D600. The minimum Cph concentration for effect lay between 1 and 5 × 10-7M, and the effects of Cph 2 × 10-6M (approximately the ID50) were totally antagonized by 12.8 mM Ca.
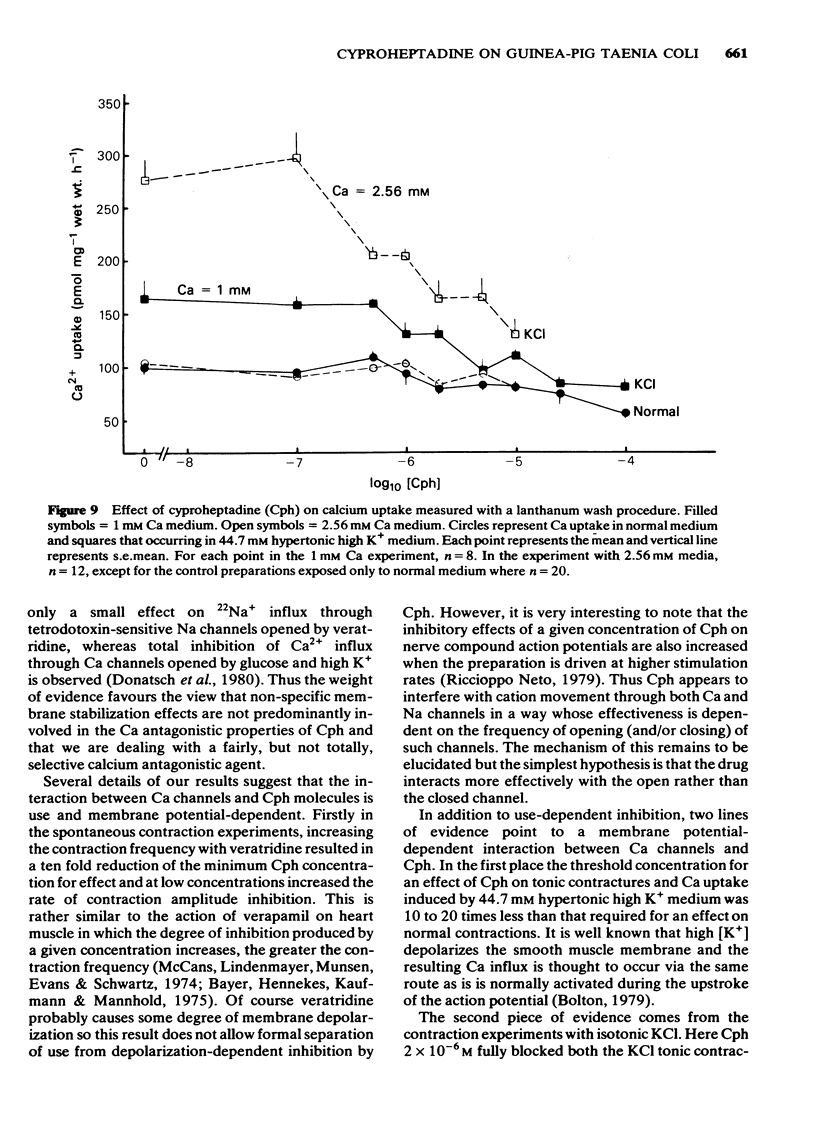
7 By means of a lanthanum wash procedure, Cph ≥ 2 × 10-6M was found to decrease the 45Ca uptake occurring into strips of taenia coli in normal medium, although the maximum effect (at Cph 10-5M) amounted to only 25% inhibition of the uptake occurring into control strips (also found with D600). The increased uptake occurring in hypertonic 44.7 mM high-potassium medium was inhibited in a dose-dependent manner by Cph 1 × 10-7M.
8 The results are consistent with an action of Cph in reducing the flow of Ca2+ through voltage-dependent Ca channels in the smooth muscle cell membrane. It is suggested that the interaction of Cph molecules with such sites is dependent upon membrane potential as well as drug concentration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBRING E. Membrane potentials of smooth muscle fibres of the taenia coli of the guinea-pig. J Physiol. 1954 Aug 27;125(2):302–315. doi: 10.1113/jphysiol.1954.sp005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer R., Hennekes R., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. I. Pattern of inotropic effects of the racemic compounds. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):49–68. doi: 10.1007/BF00499989. [DOI] [PubMed] [Google Scholar]
- Boev K., Golenhofen K. Sucrose-gap technique with pressed-rubber membranes. Pflugers Arch. 1974 Jul 9;349(3):277–283. doi: 10.1007/BF00592455. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Widdicombe J. H. The use of lanthanum to estimate the numbers of extracellular cation-exchanging sites in the guinea-pig's taenia coli, and its effects on transmembrane monovalent ion movements. J Physiol. 1977 Apr;266(2):255–273. doi: 10.1113/jphysiol.1977.sp011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatsch P., Lowe D. A., Richardson B. P., Taylor P. Mechanism by which cyproheptadine inhibits insulin secretion. Br J Pharmacol. 1980 Nov;70(3):355–362. doi: 10.1111/j.1476-5381.1980.tb08710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Effect of potassium on the mechanical activity of taenia coli, uterus and portal vein of the guinea-pig. Q J Exp Physiol Cogn Med Sci. 1978 Apr;63(2):125–146. doi: 10.1113/expphysiol.1978.sp002426. [DOI] [PubMed] [Google Scholar]
- Golenhofen K., Lammel E. Selective suppression of some components of spontaneous activity in various types of smooth muscle by iproveratril (Verapamil). Pflugers Arch. 1972;331(3):233–243. [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Beckmann J., Holze S., Lenzen S., Poser W., Hasselblatt A. Inhibition of insulin and glucagon release from the perfused rat pancreas by cyproheptadine (Periactinol, Nuran). Diabetologia. 1976 Jul;12(3):201–206. doi: 10.1007/BF00422086. [DOI] [PubMed] [Google Scholar]
- Karaki H., Ganeshanandan S. S., Ikeda M., Urakawa N. Changes in tension Ca movement and metabolism of guinea pig taenia coli in varying concentrations of external Na and K. Jpn J Pharmacol. 1969 Dec;19(4):569–577. doi: 10.1254/jjp.19.569. [DOI] [PubMed] [Google Scholar]
- Katase T., Tomita T. Influences of sodium and calcium on the recovery process from potassium contracture in the guinea-pig taenia coli. J Physiol. 1972 Jul;224(2):489–500. doi: 10.1113/jphysiol.1972.sp009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Sutter M. C. Ouabain-induced changes in the contractile and electrical activity, potassium content, and response to drugs, of smooth muscle cells. Can J Physiol Pharmacol. 1967 May;45(3):509–520. doi: 10.1139/y67-060. [DOI] [PubMed] [Google Scholar]
- Mayer C. J., van Breemen C., Casteels T. The action of lanthanum and D600 on the calcium exchange in the smooth muscle cells of the guinea-pig Taenia coli. Pflugers Arch. 1972;337(4):333–350. doi: 10.1007/BF00586650. [DOI] [PubMed] [Google Scholar]
- McCans J. L., Lindenmayer G. E., Munson R. G., Evans R. W., Schwartz A. A dissociation of positive staircase (Bowditch) from ouabain-induced positive inotropism. Circ Res. 1974 Sep;35(3):439–447. doi: 10.1161/01.res.35.3.439. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. On the mechanism of slow calcium channel block in heart. Pflugers Arch. 1980 May;385(2):175–179. doi: 10.1007/BF00588699. [DOI] [PubMed] [Google Scholar]
- Nonomura Y., Hotta Y., Ohashi H. Tetrodotoxin and manganese ions: effects on electrical activity and tension in taenia coli of guinea pig. Science. 1966 Apr 1;152(3718):97–98. doi: 10.1126/science.152.3718.97. [DOI] [PubMed] [Google Scholar]
- Pellmar T. C., Carpenter D. O. Voltage-dependent calcium current induced by serotonin. Nature. 1979 Feb 8;277(5696):483–484. doi: 10.1038/277483a0. [DOI] [PubMed] [Google Scholar]
- Riccioppo Neto F. The local anesthetic effect of cyproheptadine on mammalian nerve fibres. Eur J Pharmacol. 1979 Mar 1;54(3):203–207. doi: 10.1016/0014-2999(79)90078-5. [DOI] [PubMed] [Google Scholar]
- Riemer J., Dörfler F., Mayer C. J., Ulbrecht G. Calcium-antagonistic effects on the spontaneous activity of guinea-pig taenia coli. Pflugers Arch. 1974;351(3):241–258. doi: 10.1007/BF00586921. [DOI] [PubMed] [Google Scholar]
- Sadovsky E., Dora A., Pfeifer Y., Polishuk W. Z., Rachamimoff R., Sulman F. G. Effect of the serotonin antagonist cyproheptadine on uterine and duodenal muscle contractions in rats. Gynecol Invest. 1973;4(3):140–147. doi: 10.1159/000301717. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrophysiology of mammalian smooth muscle. Prog Biophys Mol Biol. 1975;30(2-3):185–203. doi: 10.1016/0079-6107(76)90009-2. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Aaronson P., Loutzenhiser R. Sodium-calcium interactions in mammalian smooth muscle. Pharmacol Rev. 1978 Jun;30(2):167–208. [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]


