Abstract
1 The effects of noradrenaline (NA) and isoprenaline on isolated atria from aorta-coarcted hypertensive rats (AHR) at early (6 day) and chronic (28 day) stages of hypertension were studied and compared with time-matched, sham-operated, normotensive rats (SNR). The number and affinity of beta-adrenoceptor ((-)-[3H]-dihydroalprenolol binding sites) were also studied in cardiac membranes prepared from these animals. 2 Six and 28 days after complete ligation of the abdominal aorta between the two renal arteries, rats became hypertensive with significantly greater arterial blood pressures than time-matched SNR. 3 At both stages of hypertension, the atrial inotropic or chronotropic effects of NA and isoprenaline from hypertensive rats were similar to time-matched SNR. Moreover, no differences in atrial reactivity were observed between the early and chronic stages of hypertension. 4 Irrespective of the stage of hypertension, cardiac membranes from the AHR contained the same number of beta-adrenoceptors as time-matched SNR. In addition, the receptor affinity for the radioligand within each group was equivalent. However, the chronic stage hypertensive rats and their time-matched controls contained fewer beta-adrenoceptors and these receptors had greater affinity for the radioligand when compared with cardiac membranes from rats at the early stage of hypertension and their controls. 5 The observed equivalent chronotropic and inotropic responses to NA and isoprenaline between the hypertensive and normotensive rats in both stages of hypertension may be explained in terms of similar receptor number and receptor binding affinity. 6 The reduced number of beta-adrenoceptors with greater binding affinity in day 28 normotensive or hypertensive rats may be a compensatory mechanism for these animals to maintain normal cardiac function with increasing age.
Full text
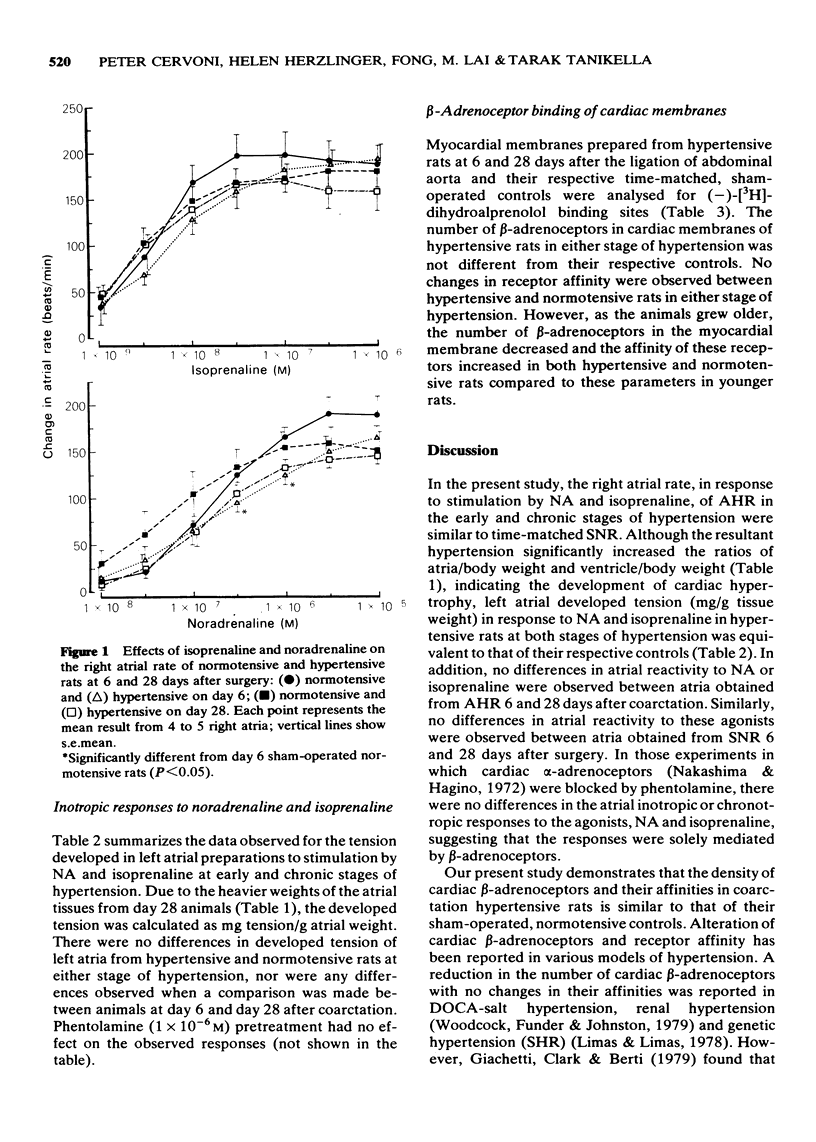
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. P., Potter L. T. Cardiac beta-adrenoceptors during normal growth of male and female rats. Br J Pharmacol. 1980 Jan;68(1):65–70. doi: 10.1111/j.1476-5381.1980.tb10699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M., Kuchii M., Shibata S. Differences of cardiac reactivity between spontaneously hypertensive and normotensive rats. Eur J Pharmacol. 1972 Jul;19(1):1–11. doi: 10.1016/0014-2999(72)90070-2. [DOI] [PubMed] [Google Scholar]
- Giachetti A., Clark T. L., Berti F. Subsensitivity of cardiac beta-adrenoceptors in renal hypertensive rats. J Cardiovasc Pharmacol. 1979 Jul-Aug;1(4):467–471. doi: 10.1097/00005344-197907000-00009. [DOI] [PubMed] [Google Scholar]
- Greenberg L. H., Weiss B. beta-Adrenergic receptors in aged rat brain: reduced number and capacity of pineal gland to develop supersensitivity. Science. 1978 Jul 7;201(4350):61–63. doi: 10.1126/science.208145. [DOI] [PubMed] [Google Scholar]
- Hallbäck M., Lundgren Y., Weiss L. Reactivity to noradrenaline of aortic strips and portal veins from spontaneously hypertensive and normotensive rats. Acta Physiol Scand. 1971 Feb;81(2):176–181. doi: 10.1111/j.1748-1716.1971.tb04890.x. [DOI] [PubMed] [Google Scholar]
- Kunos G., Robertson B., Kan W. H., Preiksaitis H., Mucci L. Adrenergic reactivity of the myocardium in hypertension. Life Sci. 1978 Mar;22(10):847–854. doi: 10.1016/0024-3205(78)90608-2. [DOI] [PubMed] [Google Scholar]
- Lai F. M., Tanikella T., Thibault L., Chan P. S., Cervoni P. Effects of different stages of aortic coarctation hypertension on aortic contraction and relaxation in rats. J Pharmacol Exp Ther. 1980 Aug;214(2):388–394. [PubMed] [Google Scholar]
- Limas C., Limas C. J. Reduced number of beta-adrenergic receptors in the myocardium of spontaneously hypertensive rats. Biochem Biophys Res Commun. 1978 Jul 28;83(2):710–714. doi: 10.1016/0006-291x(78)91047-1. [DOI] [PubMed] [Google Scholar]
- Nakashima M., Hagino Y. Evidence for the existence of alpha adrenergic receptor in isolated rat atria. Jpn J Pharmacol. 1972 Apr;22(2):227–233. doi: 10.1254/jjp.22.227. [DOI] [PubMed] [Google Scholar]
- Rojo-Ortega J. M., Genest J. A method for production of experimental hypertension in rats. Can J Physiol Pharmacol. 1968 Nov;46(6):883–885. doi: 10.1139/y68-137. [DOI] [PubMed] [Google Scholar]
- Shibata S., Kurahashi K., Kuchii M. A possible etiology of contractility impairment of vascular smooth muscle from spontaneously hypertensive rats. J Pharmacol Exp Ther. 1973 May;185(2):406–417. [PubMed] [Google Scholar]
- Woodcock E. A., Funder J. W., Johnston C. I. Decreased cardiac beta-adrenergic receptors in deoxycorticosterone-salt and renal hypertensive rats. Circ Res. 1979 Oct;45(4):560–565. doi: 10.1161/01.res.45.4.560. [DOI] [PubMed] [Google Scholar]
- Woodcock E. A., Funder J. W., Johnston C. I. Decreased cardiac beta-adrenoceptors in hypertensive rats. Clin Exp Pharmacol Physiol. 1978 Sep-Oct;5(5):545–550. doi: 10.1111/j.1440-1681.1978.tb00709.x. [DOI] [PubMed] [Google Scholar]


