Abstract
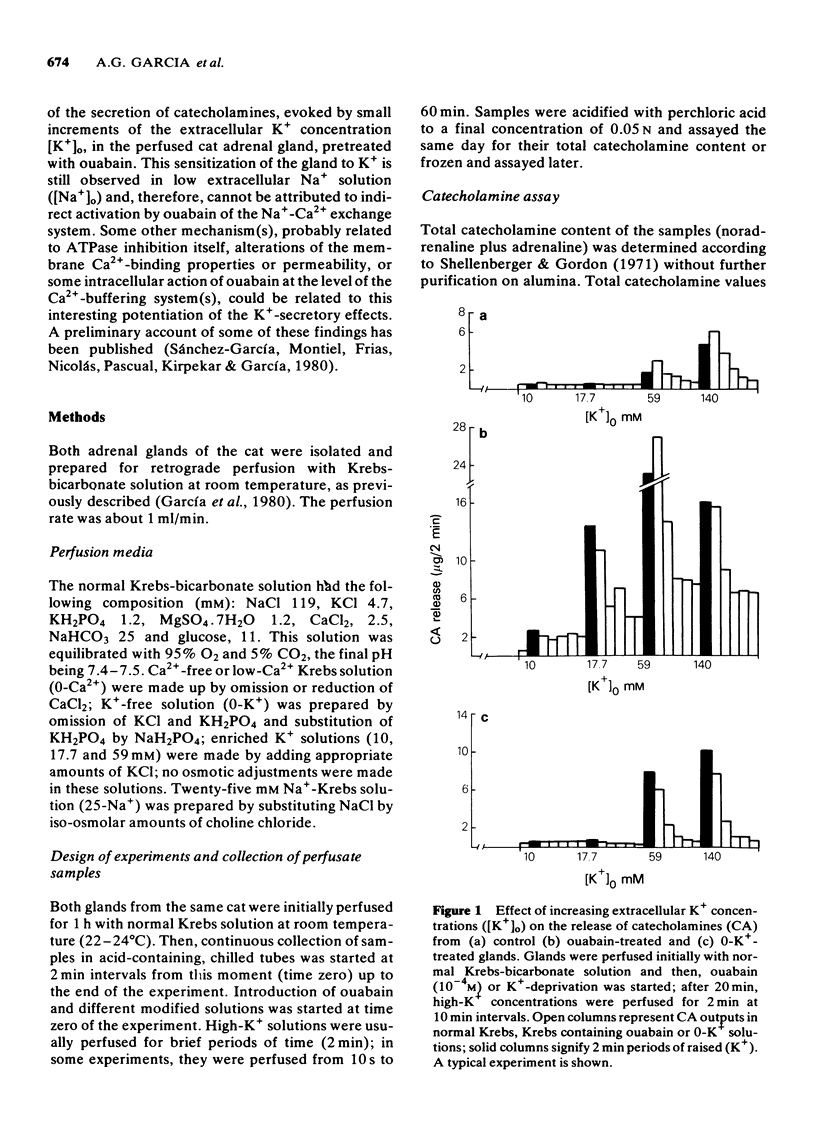
1 A vigorous catecholamine secretory response was evoked by small increments (2-10 mM) of the extracellular concentration of K+ ([K+])o) in cat adrenal glands treated with ouabain (10(-4) M), and perfused with Krebs-bicarbonate solution at room temperature. 2 The secretory response depends on [K+]o; increments of [K+]o as small as 2 mM for 2 min evoked a clear secretory response; at 10-17.7 mM K+, the maximal secretory response was observed. In normal glands, not treated with ouabain, no increase of the rate of catecholamine output was observed by raising [K+]o up to 17.7 mM for 2 min. 3 The K+ secretory response was time-dependent, requiring at least 1 min to be initiated; on continued exposure to 10 mM [K+]o, the enhanced response remained for at least 1 h. 4 In low [Na+]o, the K+-secretory response was unchanged. However, in 0-Ca2+, high-Mg2+ solutions, or in the presence of D600, an organic Ca2+ antagonist, it was abolished. 5 The K+-induced secretory response was not altered in the presence of tetrodoxin or tetraethylammonium. 6 It is concluded that ouabain potentiated the catecholamine secretory response to raised [K+]o by increasing the amount of Ca2+ available to the secretory machinery through (a) mobilization of an enhanced pool of membrane-bound Ca2+, (b) activation of membrane Ca2+ inward current; or (c) decrease of intracellular Ca2+ buffering systems. The activation by ouabain of a membrane Na+-Ca2+ exchange system is not involved in this K+-secretory response. It is suggested that the plasma membrane ATPase enzyme system, by changing the affinity of its Ca2+ binding sites, might control the availability of this cation to the secretory machinery and, therefore, modulate catecholamine secretion in the adrenal gland.
Full text
PDF
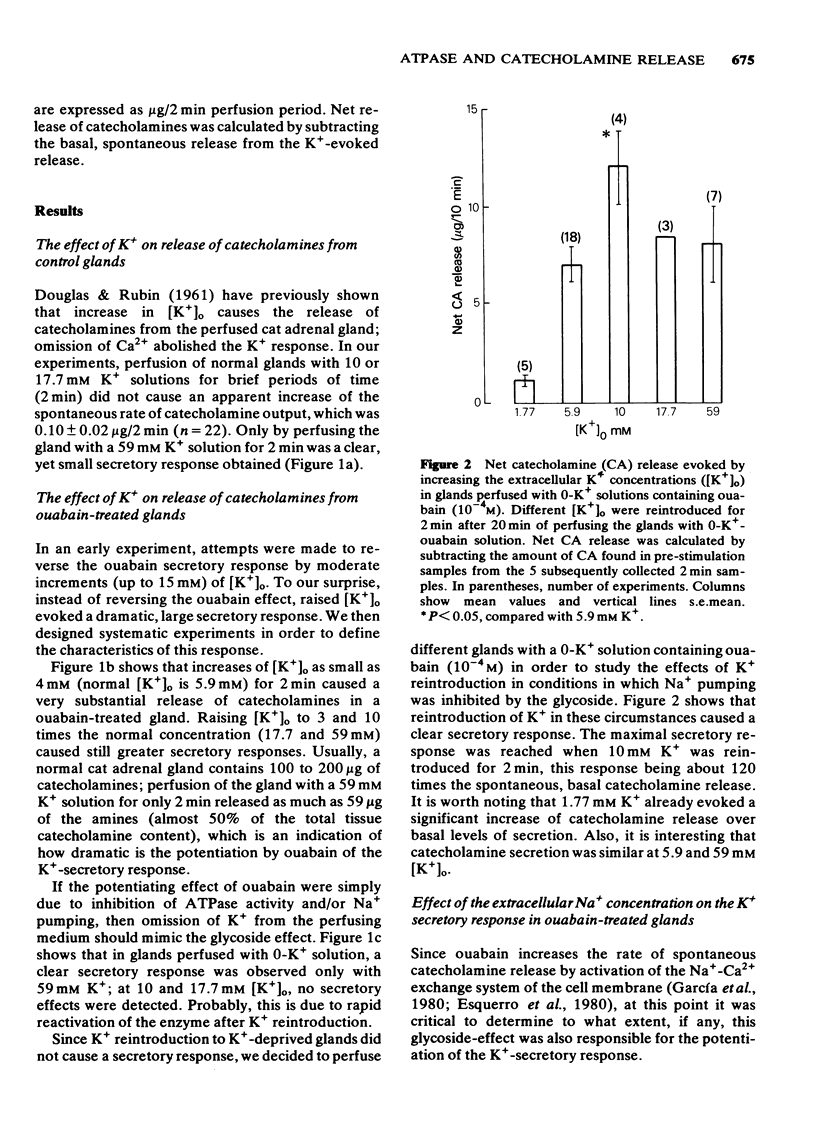
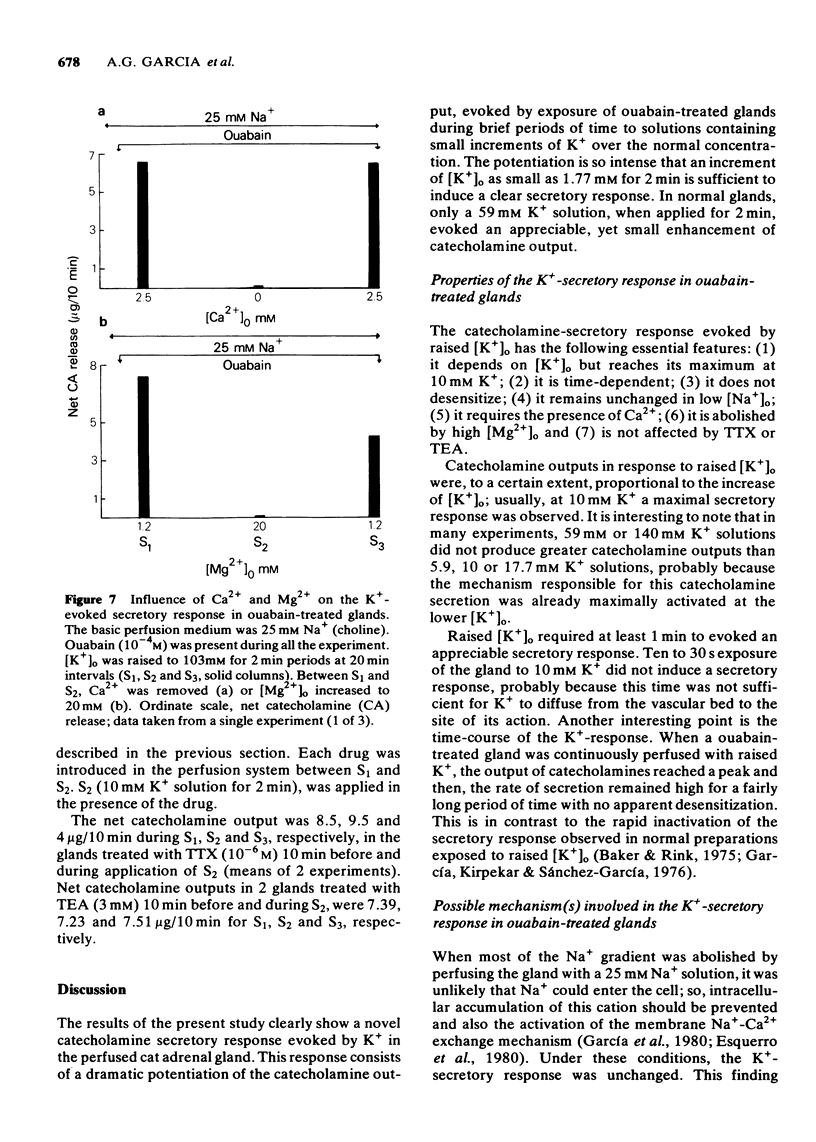


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aunis D., García A. G. Correlation between catecholamine secretion from bovine isolated chromaffin cells and [3H]-ouabain binding to plasma membranes. Br J Pharmacol. 1981 Jan;72(1):31–40. doi: 10.1111/j.1476-5381.1981.tb09101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks P. The effect of ouabain on the secretion of catecholamines and on the intracellular concentration of potassium. J Physiol. 1967 Dec;193(3):631–637. doi: 10.1113/jphysiol.1967.sp008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L., Harder D., Sperelakis N., Rubio R., Berne R. M. Cardiac glycoside stimulation of inward Ca++ current in vascular smooth muscle of canine coronary artery. J Pharmacol Exp Ther. 1979 Apr;209(1):62–66. [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
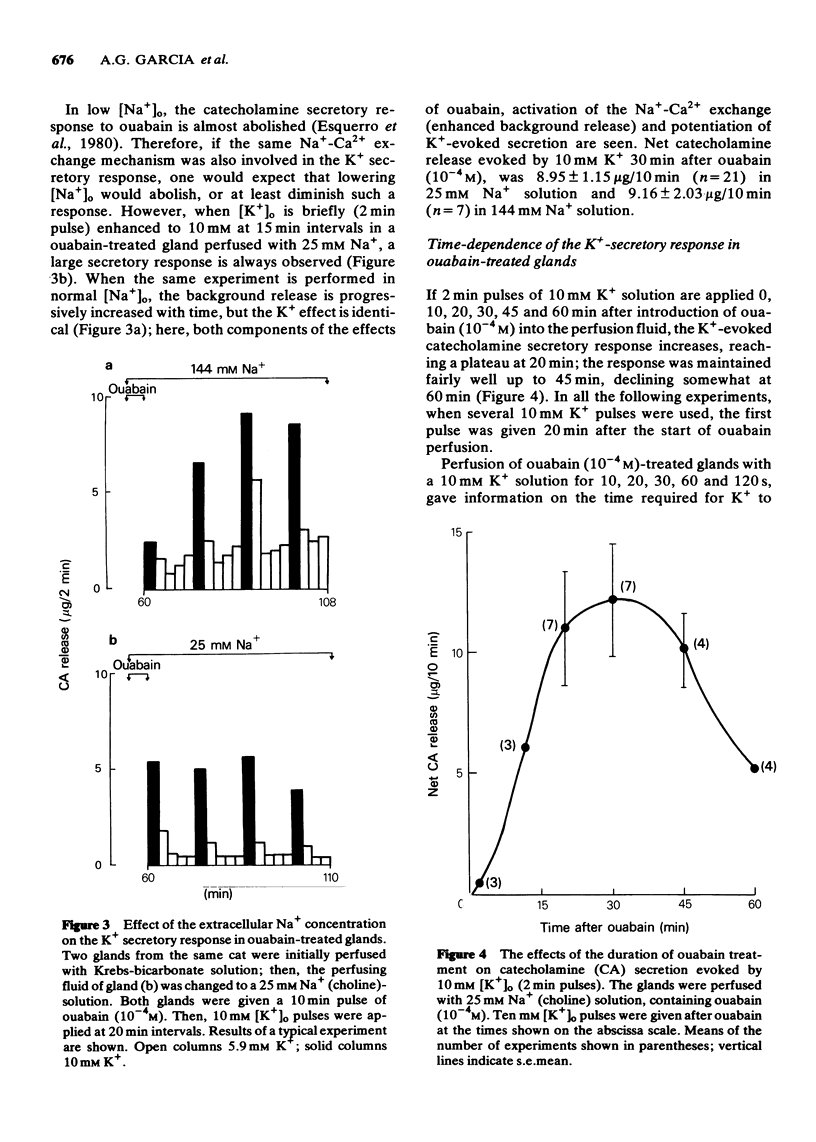
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerro E., Garcia A. G., Herandez M., Kirpekar S. M., Prat J. C. Catecholamine secretory response to calcium reintroduction in the perfused cat adrenal gland treated with ouabain. Biochem Pharmacol. 1980 Oct 1;29(19):2669–2673. doi: 10.1016/0006-2952(80)90084-2. [DOI] [PubMed] [Google Scholar]
- Fiskum G., Lehninger A. L. The mechanisms and regulation of mitochondrial Ca2+ transport. Fed Proc. 1980 May 15;39(7):2432–2436. [PubMed] [Google Scholar]
- GROSSMAN A., FURCHGOTT R. F. THE EFFECTS OF FREQUENCY OF STIMULATION AND CALCIUM CONCENTRATION ON CA45 EXCHANGE AND CONTRACTILITY ON THE ISOLATED GUINEA-PIG AURICLE. J Pharmacol Exp Ther. 1964 Jan;143:120–130. [PubMed] [Google Scholar]
- Garcia A. G., Garcia-Lopez E., Montiel C., Nicolas G. P., Sanchez-Garcia P. Correlation between catecholamine release and sodium pump inhibition in the perfused adrenal gland of the cat. Br J Pharmacol. 1981 Nov;74(3):665–672. doi: 10.1111/j.1476-5381.1981.tb10477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
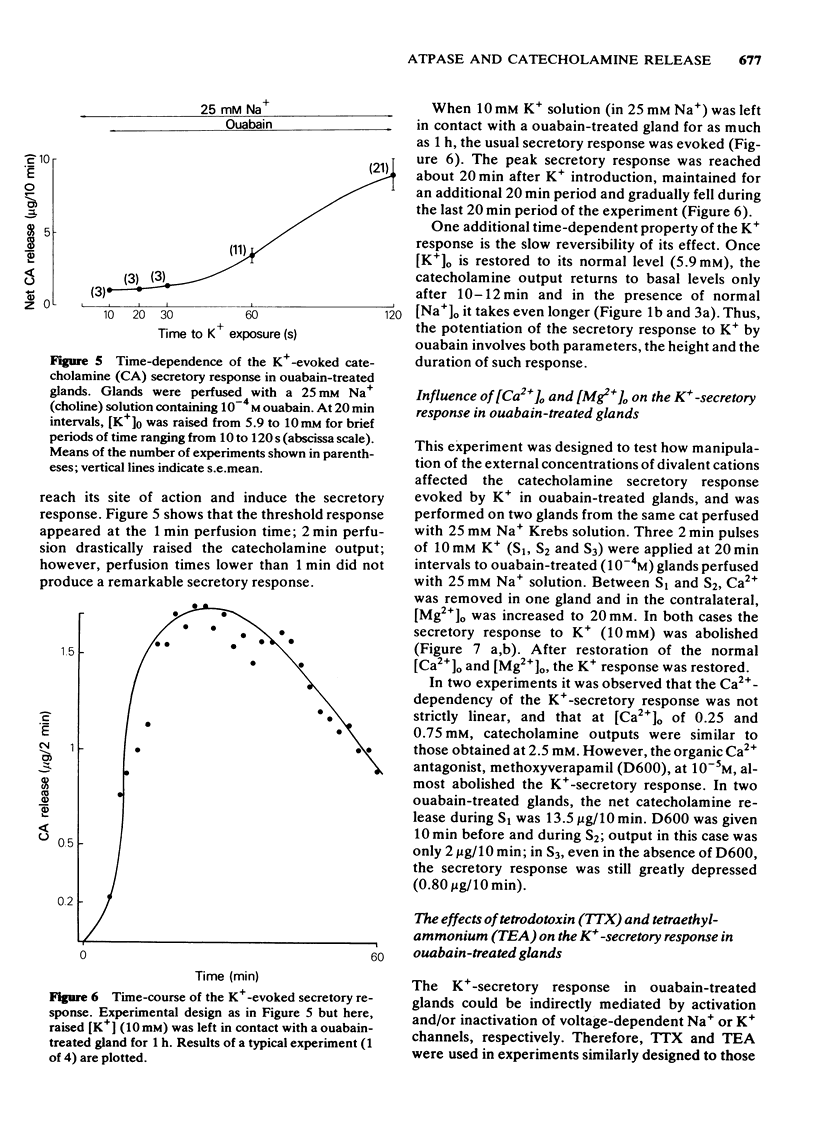
- Garcia A. G., Hernandez M., Horga J. F., Sanchez-Garcia P. On the release of catecholamines and dopamine-beta-hydroxylase evoked by ouabain in the perfused cat adrenal gland. Br J Pharmacol. 1980 Mar;68(3):571–583. doi: 10.1111/j.1476-5381.1980.tb14573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M., Sanchez-Garcia P. Release of noradrenaline from the cat spleen by nerve stimulation and potassium. J Physiol. 1976 Oct;261(2):301–317. doi: 10.1113/jphysiol.1976.sp011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais A., Lane L. K., Anner B. M., Lindenmayer G. E., Schwartz A. A possible molecular mechanism of the action of digitalis: ouabain action on calcium binding to sites associated with a purified sodium-potassium-activated adenosine triphosphatase from kidney. Circ Res. 1977 Jan;40(1):8–14. doi: 10.1161/01.res.40.1.8. [DOI] [PubMed] [Google Scholar]
- Greenspan A. M., Morad M. Electromechanical studies on the inotropic effects of acetylstrophanthidin in ventricular muscle. J Physiol. 1975 Dec;253(2):357–384. doi: 10.1113/jphysiol.1975.sp011194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Kanno T. Influences of extracellular calcium and potassium concentrations on adrenaline release and membrane potential in the perfused adrenal medulla of the rat. Jpn J Physiol. 1978;28(3):275–289. doi: 10.2170/jjphysiol.28.275. [DOI] [PubMed] [Google Scholar]
- Orkand R. K. Extracellular potassium accumulation in the nervous system. Fed Proc. 1980 Apr;39(5):1515–1518. [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]
- Weingart R., Kass R. S., Tsien R. W. Is digitalis inotropy associated with enhanced slow inward calcium current? Nature. 1978 Jun 1;273(5661):389–392. doi: 10.1038/273389a0. [DOI] [PubMed] [Google Scholar]


