Abstract
1 Effects of organic Ca2+-antagonists, verapamil and diltiazem, and cations, Ni2+, Mn2+, Co2+ and La3+ on Ca2+ current (ICa) separated from other ionic currents in a Helix neurone were studied. A suction pipette technique which allows internal perfusion of the cell body and voltage clamp was used.
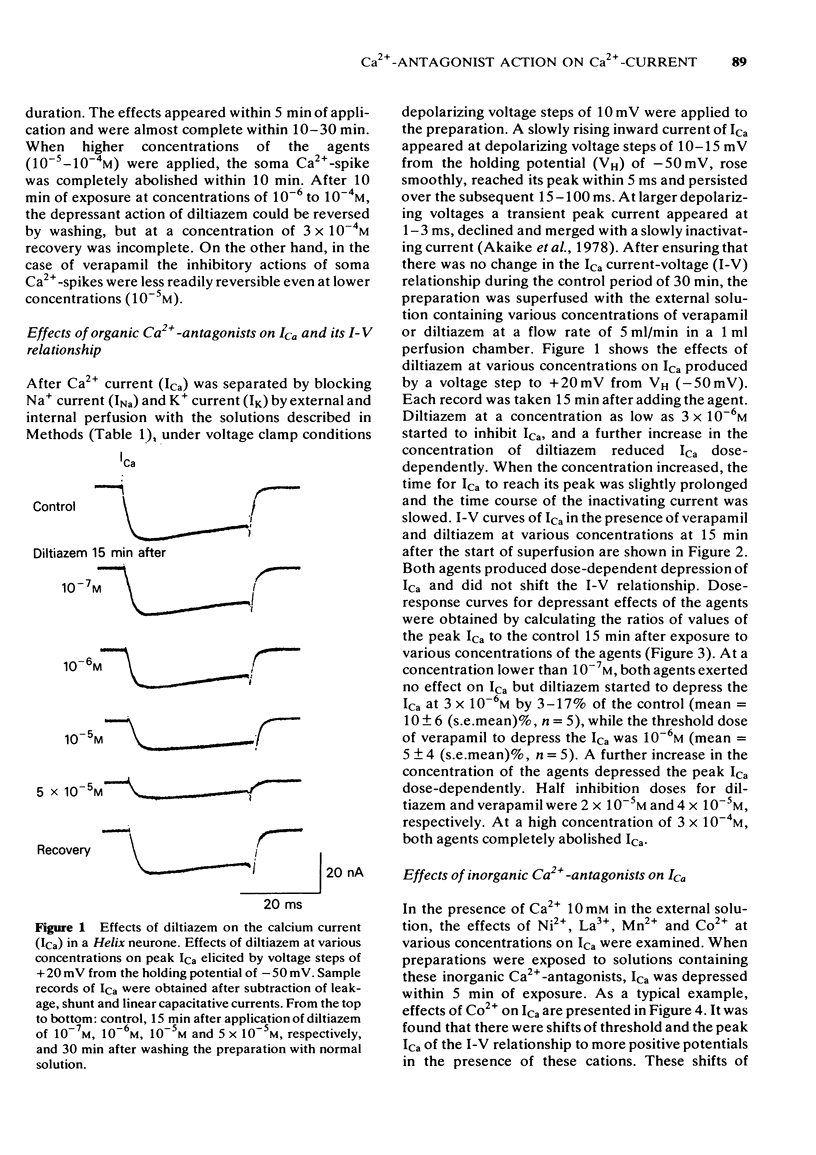
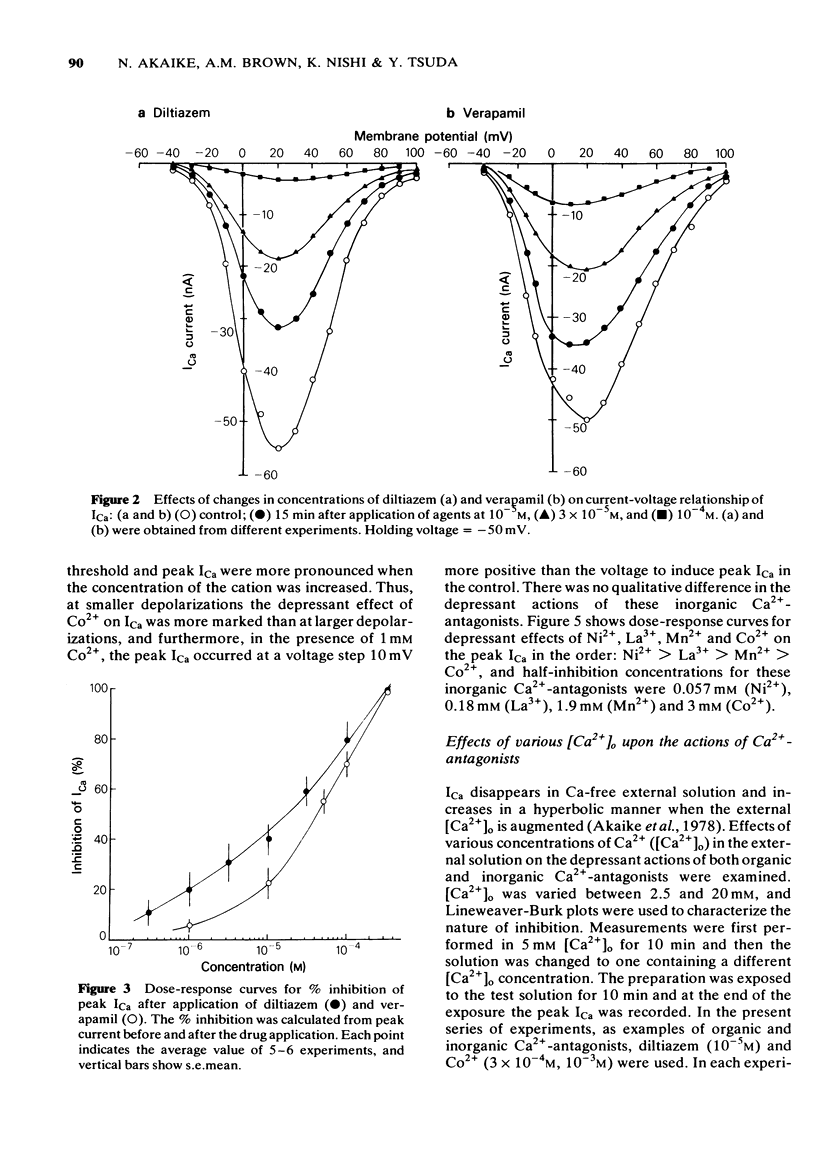
2 Verapamil and diltiazem (10-6-10-4 M) increased the threshold, and decreased both the amplitude and rate of rise of the soma Ca2+-spike. Both agents inhibited ICa over the entire range of the current-voltage (I-V) relationship dose-dependently, without shifting the threshold of the I-V relationship. Increases in external Ca2+ overcame the inhibitory action of the agents.
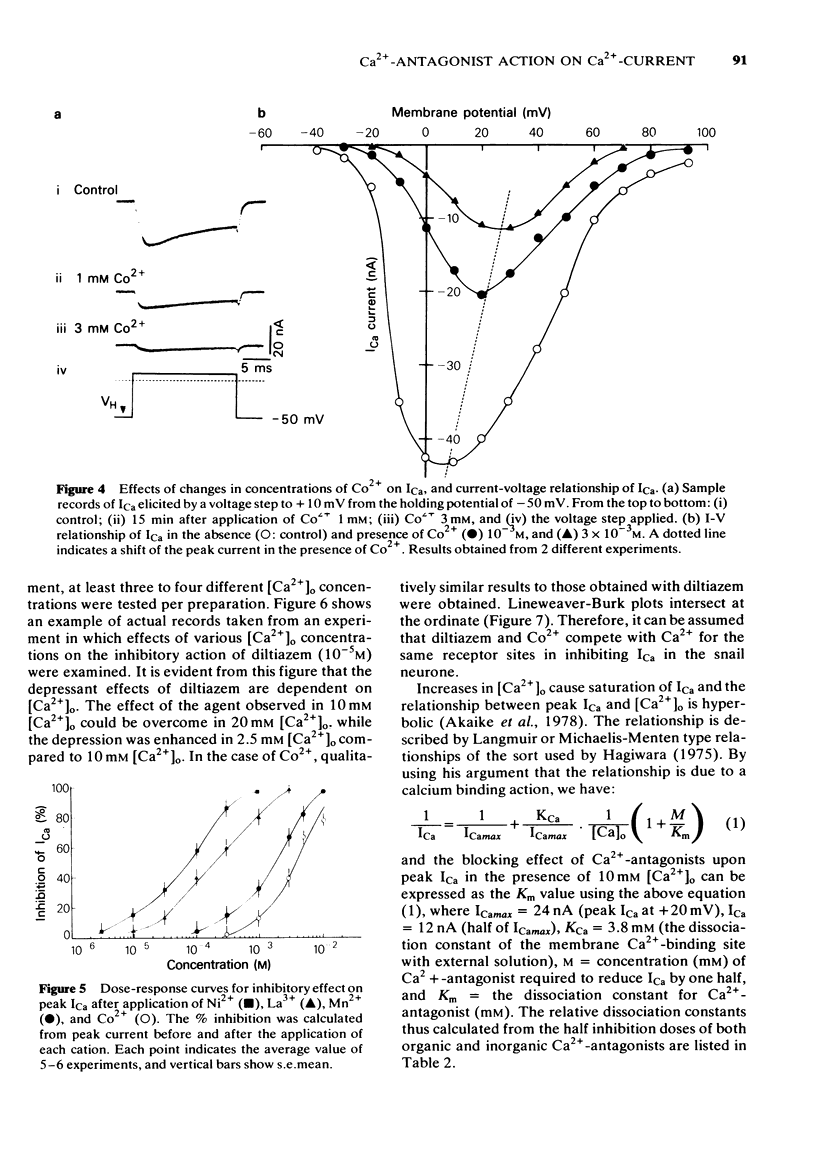
3 Divalent cations, Ni2+, Mn2+, Co2+ and the trivalent cation, La3+ inhibited ICa dose-dependently, but induced shifts of the I-V relationship to more positive voltages. The order of potency of inhibition of ICa among these cations was as follows; Ni2+ > La3+ > Mn2+ > Co2+.
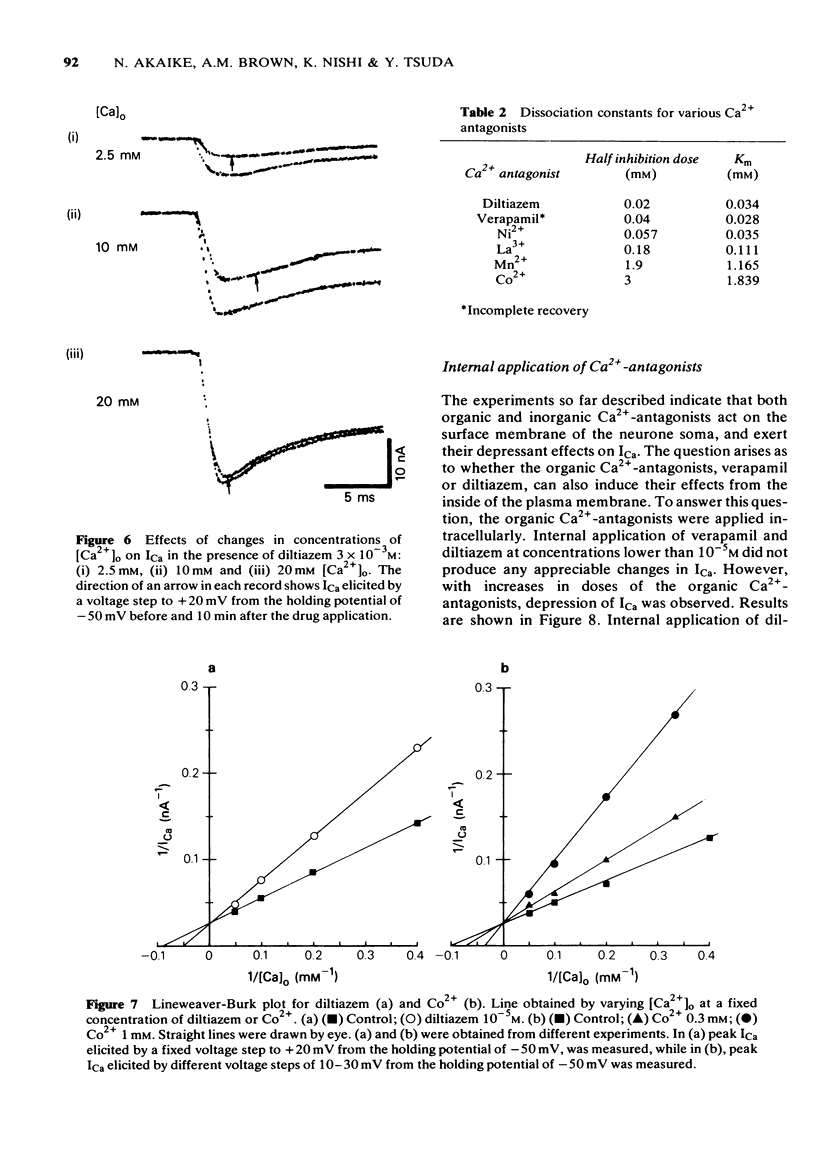
4 Double reciprocal plots for peak ICa versus external Ca2+ concentrations in the presence or absence of both organic and inorganic Ca2+-antagonists intersect at the ordinate. Results indicate that both organic and inorganic Ca2+-antagonists compete for Ca2+ at the common binding site for Ca2+.
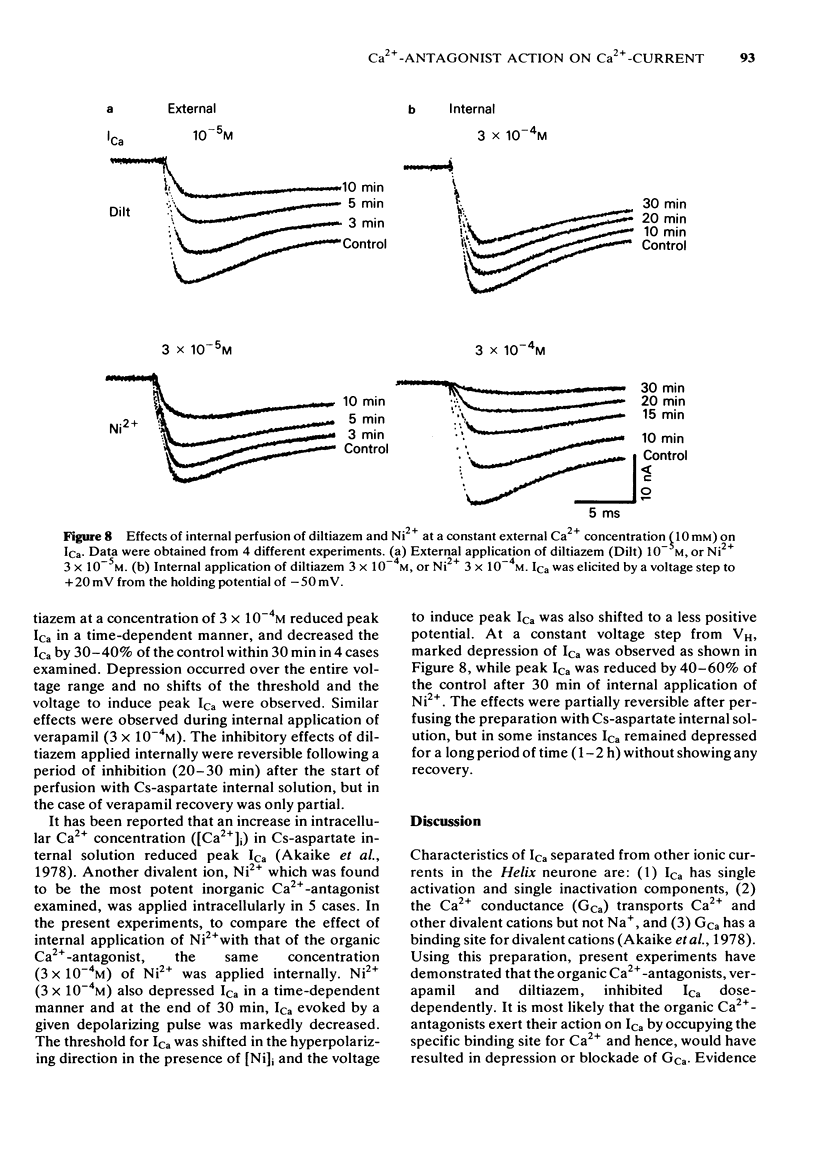
5 Internal application of the organic Ca2+-antagonists (10-4 M) inhibited ICa in a time-dependent manner to about 40-60% of the control. Ni2+, when applied internally, also depressed ICa.
6 The results provide evidence that organic Ca2+-antagonists occupy the binding site for Ca2+ in a competitive manner at the surface of the soma membrane of the Helix neurone, while divalent and trivalent cations, in addition to inhibiting ICa in a similar manner to the organic Ca2+-antagonists, change the surface charge of the soma membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Nishi K., Oyama Y. Inhibitory effects of propranolol on the calcium current of Helix neurones. Br J Pharmacol. 1981 Jun;73(2):431–434. doi: 10.1111/j.1476-5381.1981.tb10439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer R., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. II. Pattern of inotropic effects of the optical isomers. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):69–80. doi: 10.1007/BF00499990. [DOI] [PubMed] [Google Scholar]
- Bayer R., Rodenkirchen R., Kaufmann R., Lee J. H., Hennekes R. The effects of nifedipine on contraction and monophasic action potential of isolated cat myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1977 Dec;301(1):29–37. doi: 10.1007/BF00501261. [DOI] [PubMed] [Google Scholar]
- Geduldig D., Gruener R. Voltage clamp of the Aplysia giant neurone: early sodium and calcium currents. J Physiol. 1970 Nov;211(1):217–244. doi: 10.1113/jphysiol.1970.sp009276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca-dependent action potential. Membranes. 1975;3:359–381. [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Mnich Z. Studies on the inhibitory effect of verapamil on the slow inward current in mammalian ventricular myocardium. J Mol Cell Cardiol. 1978 Nov;10(11):1037–1052. doi: 10.1016/0022-2828(78)90400-5. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Effect of internal fluoride and phosphate on membrane currents during intracellular dialysis of nerve cells. Nature. 1975 Oct 23;257(5528):691–693. doi: 10.1038/257691a0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G. N., Ochsenfeld M. M. Studies on the ionic permeability of muscle cells and their models. Biophys J. 1965 Nov;5(6):777–807. doi: 10.1016/S0006-3495(65)86752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Maylie J. Calcium and cardiac electrophysiology. Some experimental considerations. Chest. 1980 Jul;78(1 Suppl):166–173. [PubMed] [Google Scholar]
- Nagao T., Sato M., Nakajima H., Kiyomoto A. Studies on a new 1, 5-benzothiazepine derivative (CRD-401). II. Vasodilator actions. Jpn J Pharmacol. 1972 Feb;22(1):1–10. doi: 10.1254/jjp.22.1. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Hoshiyama M., Yamashita K., Kiyomoto A. Effect of diltiazem on electrical and mechanical activity of isolated cardiac ventricular muscle of guinea pig. Jpn J Pharmacol. 1975 Aug;25(4):383–392. doi: 10.1254/jjp.25.383. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Schanne O. F., Ruiz-Ceretti E. Competition for slow channel of Ca2+, Mn2+, Verapamil, and D-600 in rat ventricular muscle? J Mol Cell Cardiol. 1980 Jun;12(6):635–638. doi: 10.1016/0022-2828(80)90020-6. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Garnier D., Gargouil Y. M., Coraboeuf E. Existence and role of a slow inward current during the frog atrial action potential. Pflugers Arch. 1969;308(2):91–110. doi: 10.1007/BF00587018. [DOI] [PubMed] [Google Scholar]
- Sato M., Nagao T., Yamaguchi I., Nakajima H., Kiyomoto A. Pharmacological studies on a new l,5-benzothiazepine derivative (CRD-401). Arzneimittelforschung. 1971 Sep;21(9):1338–1343. [PubMed] [Google Scholar]
- Standen N. B. Calcium and sodium ions as charge carriers in the action potential of an identified snail neurone. J Physiol. 1975 Jul;249(2):241–252. doi: 10.1113/jphysiol.1975.sp011013. [DOI] [PMC free article] [PubMed] [Google Scholar]


