Abstract
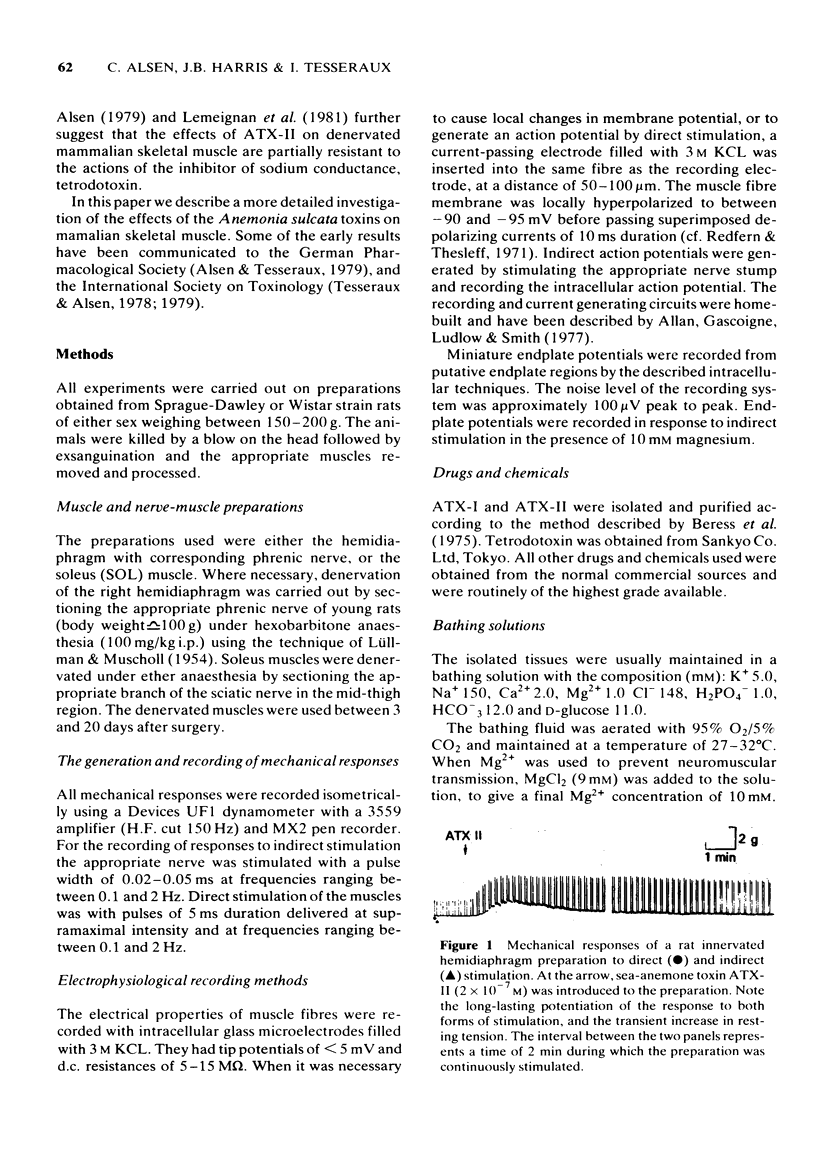
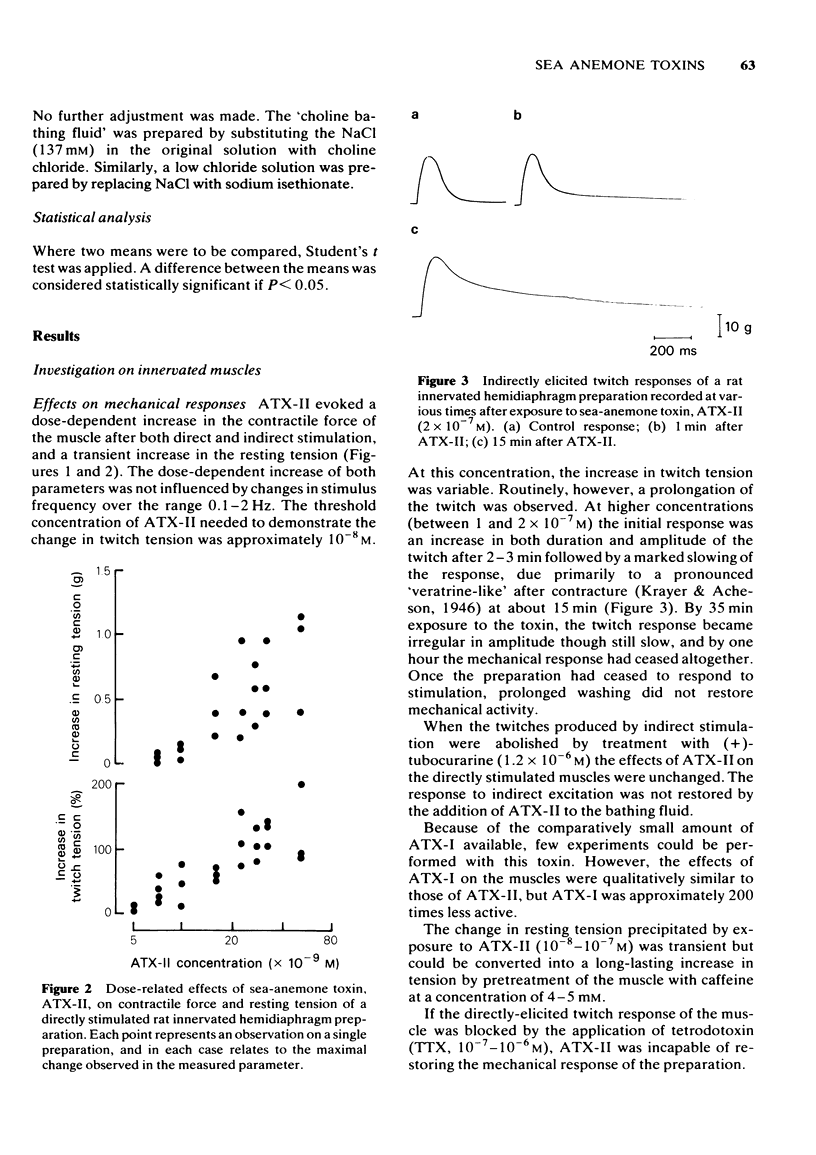
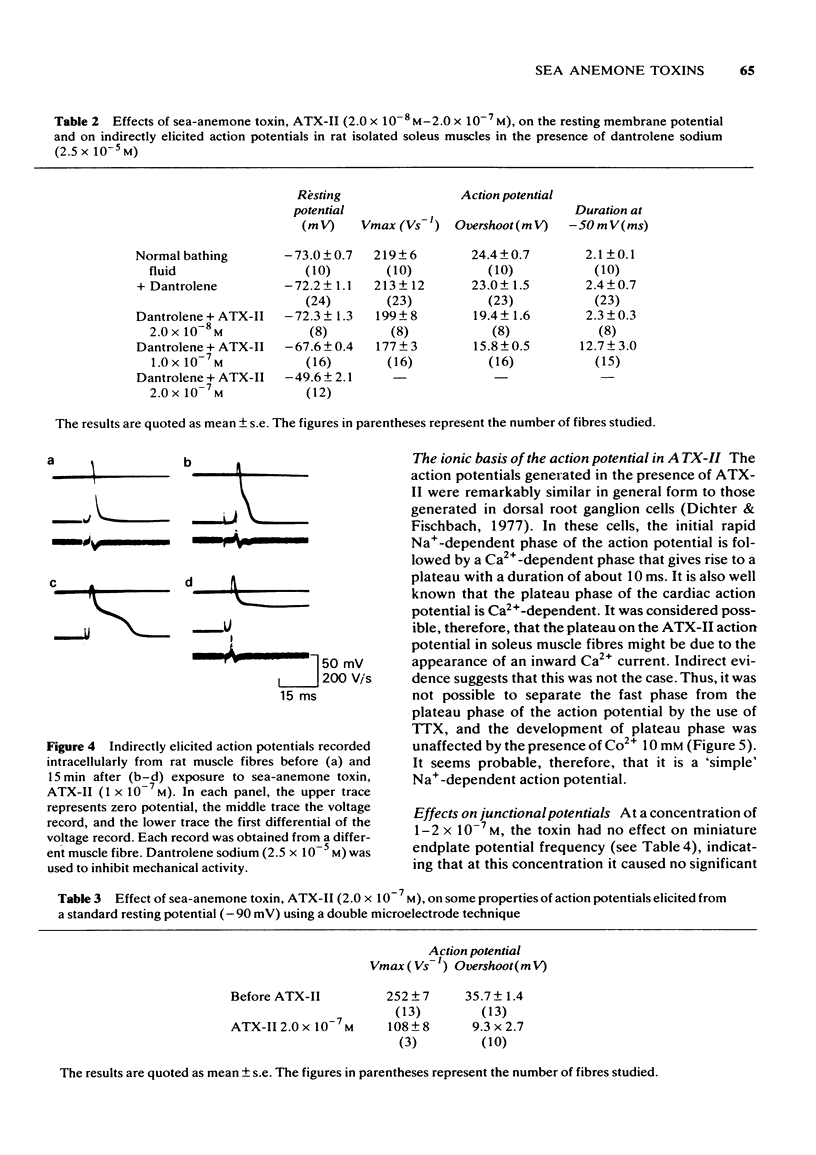
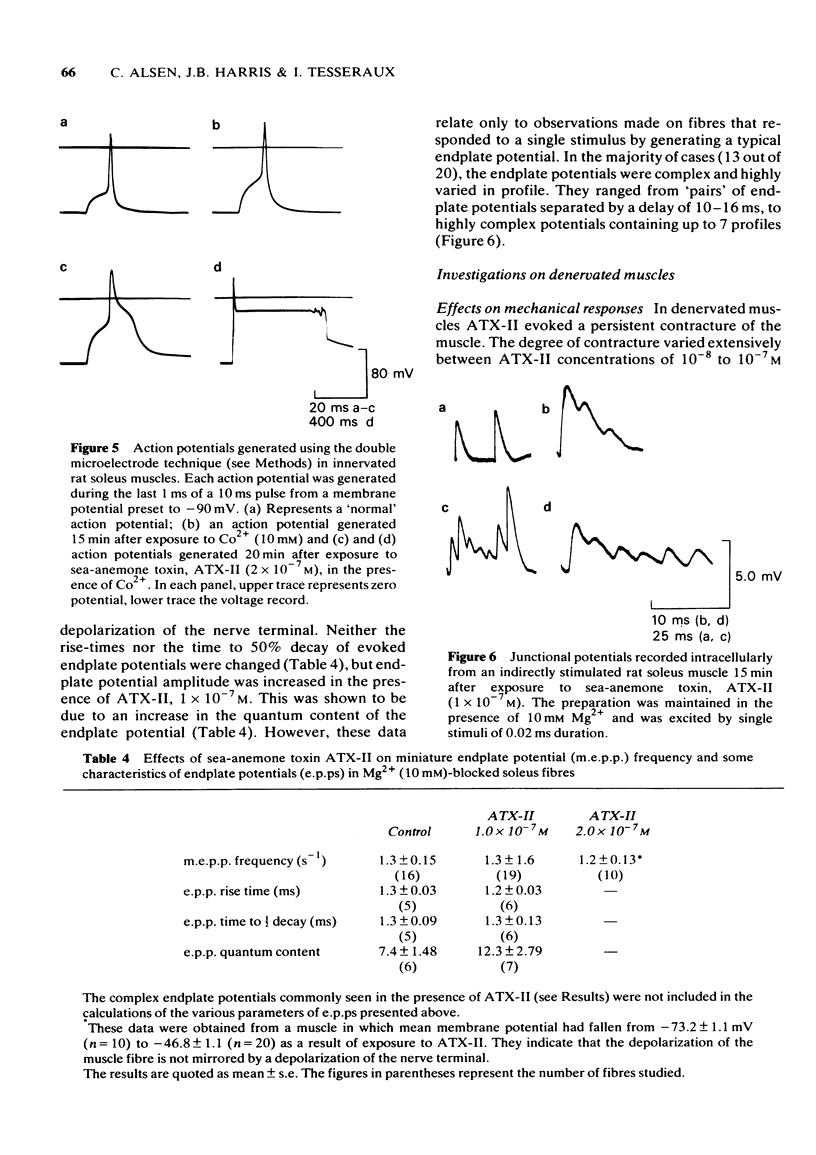
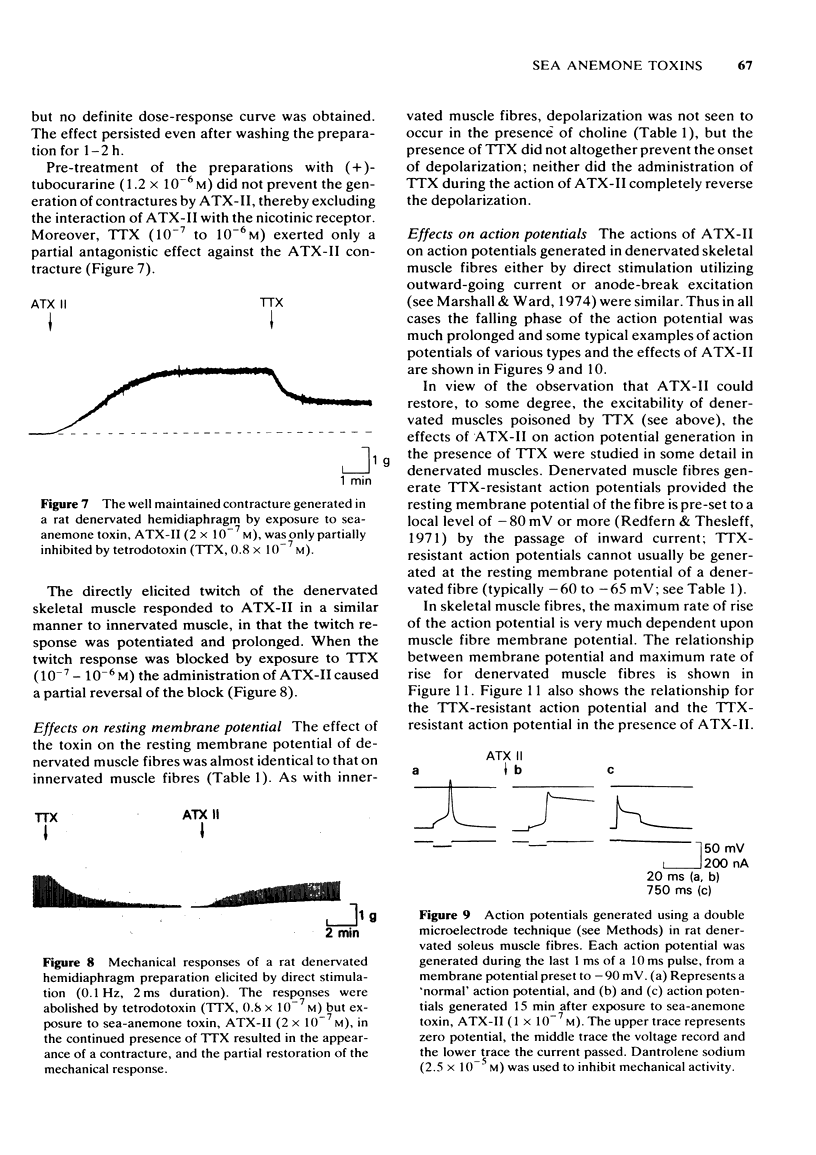
1 Some effects of the sea-anemone toxin ATX-II on mammalian nerve-muscle preparations have been described. 2 When ATX-II (10(-8)-10(-6) M) was applied to rat hemidiaphragm preparations, both directly and indirectly generated twitch responses were potentiated and prolonged. At the same time the resting tension of the preparations increased. 3 The increase in resting tension caused by ATX-II in innervated muscles was not prevented by curarization, but was reversed by exposure to tetrodotoxin. The increase in denervated muscles was not completely reversed by tetrodotoxin. 4 At concentrations exceeding 1 x 10(-7) M, ATX-II caused a sodium-dependent depolarization of both normal and denervated muscles. The depolarization of the denervated muscles was only partially reversed by tetrodotoxin. 5 In the presence of ATX-II repetitive endplate potentials (e.p.ps) were evoked by single shocks to the motor nerves in many fibres, and in those in which a single e.p.p. was still observed, the quantum content (m) was increased. Miniature e.p.p. frequency was not increased by ATX-II, even when muscle fibres were depolarized by 30 mV. 6 The indirectly and directly elicited action potentials of normal and denervated muscle fibres were much prolonged by ATX-II. The action potentials remained sodium-dependent. The sodium-dependent tetrodotoxin-resistant action potential of the denervated muscle fibre was also prolonged by ATX-II. 7 It is concluded that ATX-II both activates, and delays inactivation of, sodium channels in mammalian skeletal muscle fibres, probably in interacting with the channel "gate'.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
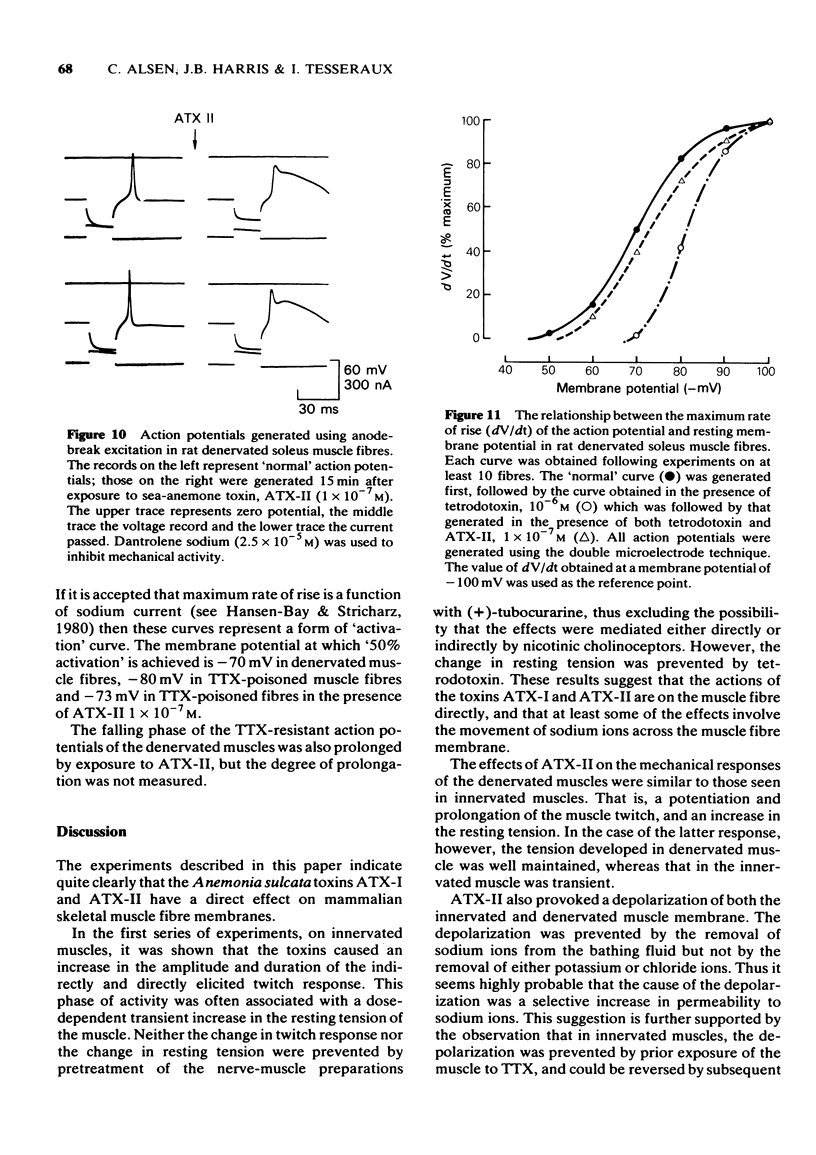
- Bay C. M., Strichartz G. R. Saxitoxin binding to sodium channels of rat skeletal muscles. J Physiol. 1980 Mar;300:89–103. doi: 10.1113/jphysiol.1980.sp013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beress L., Wunderer G., Wachter E. Amino acid sequence of toxin III from Anemonia sulcata. Hoppe Seylers Z Physiol Chem. 1977 Aug;358(8):985–988. doi: 10.1515/bchm2.1977.358.2.985. [DOI] [PubMed] [Google Scholar]
- Bergman C., Dubois J. M., Rojas E., Rathmayer W. Decreased rate of sodium conductance inactivation in the node of Ranvier induced by a polypeptide toxin from sea anemone. Biochim Biophys Acta. 1976 Nov 11;455(1):173–184. doi: 10.1016/0005-2736(76)90162-0. [DOI] [PubMed] [Google Scholar]
- Béress L., Béress R. Purification of three polypeptides with neuro- and cardiotoxic activity from the sea anemone Anemonia sulcata. Toxicon. 1975 Nov;13(5):359–367. doi: 10.1016/0041-0101(75)90196-8. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Rang H. P., Ritchie J. M. The binding of tetrodotoxin and alpha-bungarotoxin to normal and denervated mammalian muscle. J Physiol. 1974 Jul;240(1):199–226. doi: 10.1113/jphysiol.1974.sp010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A., Fischbach G. D. The action potential of chick dorsal root ganglion neurones maintained in cell culture. J Physiol. 1977 May;267(2):281–298. doi: 10.1113/jphysiol.1977.sp011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell J. N., Fairhurst A. S., Jenden D. J. Alterations of the calcium accumulating ability of striated muscle following denervation. Life Sci. 1966 Mar;5(5):439–446. doi: 10.1016/0024-3205(66)90159-7. [DOI] [PubMed] [Google Scholar]
- LULLMANN H., MUSCHOLL E. Uber die Wirkung hypotoner Salzlösungen auf das Rattenzwerchfell. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1954;221(3):209–214. [PubMed] [Google Scholar]
- Lüllmann H., Preuner J., Sunano S. On the interaction of acetylcholine, caffeine and altered ca-concentrations upon the excitation-contraction coupling in chronically denervated skeletal muscle. Pflugers Arch. 1974;352(4):279–290. doi: 10.1007/BF00585682. [DOI] [PubMed] [Google Scholar]
- Marshall M. W., Ward M. R. Anode break excitation in denervated rat skeletal muscle fibres. J Physiol. 1974 Jan;236(2):413–420. doi: 10.1113/jphysiol.1974.sp010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métézeau P., Bourneau R., Mambrini J., Tazieff-Depierre F. Action d'une neurotoxine isolée de l'anémone de mer (Anemonia sulcata) à la jonction neuromusculaire de grenouille. J Physiol (Paris) 1979;75(8):873–879. [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Romey G., Abita J. P., Schweitz H., Wunderer G., Lazdunski Sea anemone toxin:a tool to study molecular mechanisms of nerve conduction and excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4055–4059. doi: 10.1073/pnas.73.11.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F. E., Marcus P., Streng J. A. Black widow spider envenomation during pregnancy. Report of a case. Toxicon. 1979;17(2):188–189. doi: 10.1016/0041-0101(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderer G., Eulitz M. Amino-acid sequence of toxin I from Anemonia sulcata. Eur J Biochem. 1978 Aug 15;89(1):11–17. doi: 10.1111/j.1432-1033.1978.tb20890.x. [DOI] [PubMed] [Google Scholar]
- Wunderer G., Fritz H., Wachter E., Machleidt W. Amino-acid sequence of a coelenterate toxin: toxin II from Anemonia sulcata. Eur J Biochem. 1976 Sep;68(1):193–198. doi: 10.1111/j.1432-1033.1976.tb10778.x. [DOI] [PubMed] [Google Scholar]


