Abstract
Purpose
While rearing chicks in constant light (CL) inhibits anterior segment growth, these conditions also induce excessive enlargement of the vitreous chamber. The mechanisms underlying these effects are poorly understood although it has been speculated that the enlarged vitreous chambers are a product of emmetropization, a compensatory response to the altered anterior segments. We examined the ability of eyes to compensate to defocusing lenses in CL as a direct test of their ability to emmetropize. We also studied recovery responses, i.e. from lens-induced changes in CL as well as CL-induced changes alone or combined with lens-induced changes in eyes returned to normal diurnal lighting (NL).
Methods
Hatchling White-Leghorn chicks were reared in either CL or NL (control) lighting conditions (n = 36) for two weeks, with lenses of either +10 or −10 D power fitted to one eye of all chicks at the beginning of the second week. The lenses were removed at the end of the same week, at which time some CL chicks (n = 14) were shifted to NL, the rest of the chicks remaining in their respective original lighting conditiobns. Retinoscopy, IR photo-keratometry and high-frequency A-scan ultrasonography were used to track refractions, corneal radius of curvature and ocular axial dimensions respectively; data were collected on experimental days 0, 7, 9, 14 and 21.
Results
Under CL, eyes showed near normal, albeit slightly exaggerated responses to +10 D lenses while the response to −10 D lenses was disrupted. With +10 D lenses, lens-wearing eyes became more hyperopic (RE), and had shorter vitreous chambers (VC) and optical axial lengths (OL) relative to their fellows by the end of the lens period (RE: +10.5 ± 1.5 D, CL, +8.25 ± 2.5 D, NL; VC: −0.363 ± 0.129 mm, CL; −0.306 ± 0.110 mm, NL; OL: -0.493 ± 0.115 mm, CL, −0.379 ± 0.106 mm, NL (mean interocular difference ± SD). With −10 D lenses, the NL group showed a myopic shift in RE and increased elongation of both VC depth and OL (RE: −10.75 ± 2.0 D; VC depth: 0.554 ± 0.097 mm; OL: 0.746 ± 0.166 mm), while the CL group showed a small hyperopic shift in RE (+4.0 ± 6.0 D). Nonetheless, CL eyes were able to recover from lens-induced hyperopia, whether they were left in CL or returned to NL. One week of exposure to NL was sufficient to reverse the effects of 2 weeks of CL on anterior and vitreous chamber dimensions.
Conclusion
CL impairs emmetropization. Specifically, it disrupts compensation to lens-imposed hyperopia but not imposed myopia. However, CL eyes are able to recover from lens-induced hyperopia, suggesting that the mechanisms underlying the compensatory responses to defocusing lenses are different from those involved in recovery responses. The ocular growth effects of CL on young eyes are reversible under NL.
Keywords: emmetropization, constant light, chick, myopia, hyperopia
Introduction
The epidemic levels of myopia in some Asian countries (Au Eong, Tay et al. 1993), the potentially sight-threatening complications associated with myopia (Curtin 1985) and the possibility that myopia might occur as a product of emmetropization have stimulated renewed interest in the mechanisms underlying the latter.
The term, emmetropization, describes the process by which neonatal refractive errors are corrected through adjustments to eye growth. Although emmetropization has a passive component, an optical artifact of normal eye growth (Hofstetter 1969; Wallman, Gottlieb et al. 1987; Edwards 1992; Wildsoet 1997), animal studies have provided convincing evidence for an active component as well. For example, when lenses are used to impose focusing errors on the eyes of young animals, compensatory growth changes involving both the choroid and sclera follow. Chicks, the most widely used model for this research, are able to compensate for a wide range of imposed myopia and hyperopia (Schaeffel, Glasser et al. 1988; Irving, Sivak et al. 1992; Wildsoet and Wallman 1995; Nevin, Schmid et al. 1998). The bidirectional nature of these responses and their rapid onset points to an active regulatory mechanism. That young chicks are able to recover from experimentally-induced refractive errors, e.g. seen when lenses are removed after compensation has occurred, has been interpreted as further evidence for active emmetropization (Irving, Callender et al. 1995; Wildsoet and Wallman 1995).
Apart from optical defocus, the light cycle used in rearing also can influence early eye growth. Of relevance to the study reported here is the observation in chicks that constant light (CL) inhibits anterior segment growth while enhancing vitreous chamber elongation (Jensen and Matson 1957; Kinnear, Lauber et al. 1974; Li, Troilo et al. 1995), although there are strain-related differences with the Cornell strain of White-Leghorn showing exaggerated anterior segment changes (Li, Troilo et al. 1995; Stone, Lin et al. 1995; Troilo, Li et al. 1995). These ocular effects of CL are reversible, provided chicks are returned to diurnal lighting cycle (NL) at a sufficiently early age (Li, Wahl et al. 2002; Li, Wahl et al. 2004).
Understanding the influence of CL rearing on emmetropization in the chick may go part way to resolving the on-going debate over the possible causal relationship between altered light exposure and myopia in humans. For example, there is a study linking exposure to light at night in infancy and childhood myopia (Quinn, Shin et al. 1999), and another linking myopia in college students with reduced hours of sleep (darkness) (Loman, Quinn et al. 2002). However, other related studies have questioned this link (Gwiazda, Ong et al. 2000; Zadnik, Jones et al. 2000; Saw, Wu et al. 2001; Saw, Zhang et al. 2002; Guggenheim, Hill et al. 2003).
In relation to the effects of CL on emmetropization in chicks, there are 2 studies of direct relevance although their findings are inconclusive. One study by Bartmann, Schaeffel et al. (1994) reports normal compensation to both plus and minus lenses, although it is not possible to rule out sign-related changes in these responses due to the use of bilateral lenses of opposite sign. A second study by Guo et al. (1996) avoided this problem by using monocular lenses fitted to hatchling chicks. However, while only partial compensation to both minus and plus lenses was observed, these data are confounded by the lack of a pre-lens CL adaptation period as included in the Bartmann and Schaeffel study. Light is known to be an important Zeitgeber for biological rhythms, among them, ocular growth rhythms that are known to be perturbed by CL (Weiss and Schaeffel 1993). Because other experimental manipulation that alter ocular growth also appear to alter ocular growth rhythms (Weiss and Schaeffel 1993; Schmid, Papastergiou et al. 1997) (Nickla, Wildsoet et al. 1998), it is important that such rhythms be allowed time to first stabilize (free-run) under CL in testing the eye’s ability to actively emmetropize under CL.
In the study reported here, we re-examined the effect of CL on active emmetropization. We asked 3 questions: 1) is lens compensation in CL similar to that in NL, i.e. is the emmetropization process altered in chicks reared in CL, 2) can chicks reared in CL recover from lens-induced changes while still in CL and/or when returned in NL, and 3) does CL affect choroidal thickness and/or other components that contribute to eye length and if so, how reversible are these effects in chicks returned to NL. Thus we studied the ability of eyes to compensate for defocus imposed with spectacle lenses and also followed both lens-treated eyes and their fellows after lenses were removed, to see if they were able to recover from induced changes, in either CL, in relation to the lens effects, or in NL, in relation to lens- and CL-effects. The high resolution offered by high frequency A-scan ultrasonography (approximately 10 μm) (Nickla, Wildsoet et al. 1997), also allowed us to characterize the effects of CL on ocular dimensions more completely than in already published studies, which employed lower resolution methods.
Aspects of this work have been published in abstract form (Padmanabhan and Wildsoet 2004).
Methods
Animals & Treatments
A total of 36 four to six day-old White Leghorn chicks obtained from a commercial hatchery (Privett Hatchery, New Mexico) were used in this study. Food and water were available ad libitum. The chicks were allocated to one of three groups, based on the lighting conditions to which they were exposed over the course of the study. On arrival, chicks were allocated to either normal diurnal lighting conditions and open cages (NL; 12 hr light/2 hr dark cycle) or constant light (CL) and special sound- and light-proof chambers. Lighting levels in the chambers were similar to the levels in open cages, ranging from 331 – 385 lux. Chicks remained in their allocated lighting environment for a 7-day period before the start of the lens-wearing period that lasted another 7 days (days 7 through 14). The pre-lens period of 7 days allowed all light-dependent body rhythms to become either entrained (NL) or become free-running (CL). At the end of the lens-wearing period, the CL chicks either remained in CL (rCL) or were placed in diurnal lighting conditions (rNL) where they remained for another 7 days. In total, there were three different rearing conditions: NLrNL (normal lighting throughout), CLrCL (constant lighting throughout) and CLrNL (constant lighting followed by normal lighting). During the lens-wearing period, chicks wore either a +10 or –10 D lens over their left eyes. Table 1 summarizes the lens power-lighting combinations used along with the number of chicks allocated to each treatment group.
Table 1.
Summary of experimental groups with numbers of chicks per group; chicks were exposed across the 3-week study period to either constant light (CLrCL), normal diurnal lighting (NLrNL), or to CL for the first 2 weeks and then NL (CLrNL).
| Lens treatment | Total | CLrCL | CLrNL | NLrNL |
|---|---|---|---|---|
| +10 D | 19 | 6 | 7 | 6 |
| −10 D | 17 | 5 | 7 | 5 |
Measurements
Refractive error (RE), corneal radius of curvature (CR) and axial ocular dimensions were tracked over the study period using retinoscopy, keratometry (IR photo-keratometer) and high-frequency (30Hz) A-scan ultrasonography respectively. Measurements were made on experimental day 0, at the start of the entrainment period, day 7, immediately prior to lens fitting, days 9 and 14, during and at the end of the lens wearing period, and days 16 and 21, during the post-lens recovering period. All measurements were performed under gaseous anesthesia (1.5% isoflurane in oxygen).
Data analysis
Averages of values for the 2 principal meridians in the case of RE and CR data were derived for use in analyses. Analyses of ultrasonography data reported here are restricted to those components significantly affected by the treatments although measurements encompassed the dimensions of all 3 main ocular compartments of the eye, anterior chamber depth (AC depth, measured from anterior corneal surface), lens thickness (LT) and vitreous chamber depth (VC depth, measured to anterior retinal surface) as well as the thicknesses of the 3 layers of the wall of the eye at the posterior pole, i.e. retinal thickness (RT), choroidal thickness (CT) and scleral thickness (ST). An optical axial length (OL) was derived from these data as the distance from the anterior corneal surface to the anterior retinal surface (AC depth+ LT+ VC depth). Scleral cup size was estimated as the sum of VC, RT and CT. To isolate the effects of the lighting and defocus manipulations on ocular dimensions, data collected at each time point were normalized to day 0 readings (pre-normalized data), thereby eliminating normal inter-animal variations. Data are presented in the text as means ± standard deviations (SD) and shown graphically as means ± standard errors of means (SE).
Analysis of variance (factorial ANOVAs) in combination with the Fishers’ PLSD post hoc test was used to assess intergroup differences. A p-value of <0.05 was considered statistically significant.
In analyzing the lens treatment effects, the data from CLrCL and CLrNL groups were pooled. For these data, changes in both the lens-treated eyes and their fellow untreated eyes across the lens-wearing period (days 7 to 14) were calculated after further normalizing the pre-normalized data to day 7 readings, to eliminate the lighting-related differences (i.e. between CL versus NL groups), evident at the start of this period. The data for lens-treated eyes were compared across lighting conditions and inter-ocular differences in these changes were also compared across lighting conditions.
The effects of CL were separately analyzed using the untreated fellow eye data pooled across lens treatments for each lighting condition; days 7 to 21 pre-normalized data were used.
To analyze the ability of these eyes to recover from lens- and/or lighting effects, the changes across the recovery period (days 14 to 21), in both previously lens-treated eyes and their fellows were calculated after normalizing the pre-normalized data to day 14 values, to eliminate the differences between eyes on day 14. This approach allows the changes over the recovery period to be more easily compared. Inter-ocular differences in these changes were used to look for evidence of recovery from lens effects. Additional analyses used day 14 and day 21 data normalized to day zero values.
Data from the untreated fellow eyes were used to analyze the ability of eyes to recover from CL effects; here, the 16-day and 21-day data were compared, after normalizing to day 14 readings. Additional analyses used day 21 data normalized to day zero values.
The experiments described herein conform to the ARVO Statement for the Use of Animals in Research. The University of California-Berkeley Animal Care and Use committee approved all experimental protocols.
Results
Our data indicate that CL disrupts emmetropization although not all expressions of emmetropization were affected. Specifically, under CL conditions the response to plus lenses, i.e. imposed myopia, was compensatory while the response to minus lenses, i.e. imposed hyperopia, was opposite to that required for compensation overall. However, eyes were able to recover from induced hyperopia under CL conditions and they also were able to recover from the effects of CL when transferred to NL.
Responses to plus & minus lenses under CL and NL conditions
Plus lenses
The response pattern observed with +10 D lenses under CL was similar to that observed under NL and consistent with previous observations for diurnal lighting conditions (Wildsoet and Wallman 1997). In NL, there was a relative increase in choroidal thickness, and decreases in VC depth, scleral cup length and OL, the net of these changes being an increase in hyperopia that neutralized approximately 80% of the imposed myopia. That the changes in the vitreous chamber depth were largely responsible for the refractive changes is indicated by the strong correlation between the refractive changes and changes in both VC depth and OL (RE & VC depth: r = −0.937, RE & OL: r = −0.961, p<0.0001; p<0.0001). In CL, the plus lenses also resulted in significant increases in hyperopia, coupled to shorter VCs, scleral cup lengths and OLs relative to their respective fellow untreated eyes (see Figure 1, left panel; Table 2). Both groups of lens-treated eyes also had thinner lenses than their fellows, the NL group more so, while the converse was true for the effects on scleral cup length and RE (see Table 2).
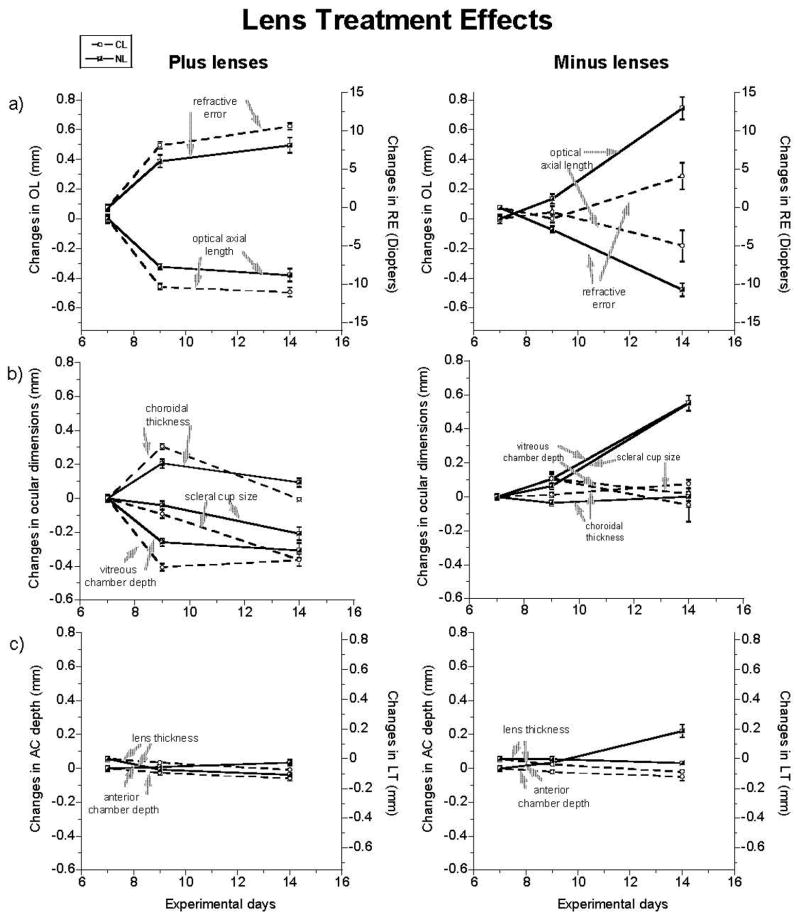
Figure 1.
The changes in interocular differences (treated eyes - fellow eyes) in ocular parameters over the lens-wearing period (left, plus lens; right, minus lens): a) refractive error (RE) and optical axial length (OL), b) vitreous chamber (VC) depth, choroidal thickness (CT) and scleral cup size, c) anterior chamber (AC) depth and lens thickness (LT). Means ± SE for pre-normalized data after further normalization to day 7 values plotted. Dashed lines with circles represent chicks in constant light (CL). Solid lines with half-shaded squares represent chicks in normal lighting cycle (NL). Plus lens-wearing eyes in both NL and CL groups developed increased hyperopia, shorter OLs and VCs. Plus lenses also increased CT in both groups but more transiently in the CL chicks. Minus lens-treated eyes developed myopia, elongated VCs and OLs, and deeper ACs in NL. Minus lenses slightly increased hyperopia in CL.
Table 2.
Mean interocular differences (± SD) in the changes in ocular parameters over the 1-week lens-wearing period, for chicks reared in constant (CL) and normal (NL) lighting. Raw data were initially normalized to day zero (pre-normalized) and then further normalized to day 7 readings to isolate the effects of the lens treatments.
| Ocular parameter | +10 D lens | −10 D lens | ||
|---|---|---|---|---|
| Constant lighting | Normal lighting | Constant lighting | Normal lighting | |
| Refractive error (D) | +10.5±1.5* | +8.25±2.5*# | +4.0±6.0* | −10.75±2.0*# |
| Corneal radius of curvature (mm) | 0.151±0.097* | 0.142±0.073* | 0.089±0.099* | −0.079±0.090# |
| Anterior chamber depth (mm) | −0.058±0.047* | 0.034±0.044# | −0.048±0.079 | 0.221±0.084*# |
| Lens thickness (mm) | −0.071±0.019* | −0.107±0.019*# | −0.086±0.025* | −0.029±0.017*# |
| Vitreous chamber depth (mm) | −0.363±0.129* | −0.306±0.110* | −0.047±0.343 | 0.554±0.097*# |
| Choroidal thickness (mm) | −0.006±0.041 | 0.094±0.062*# | 0.077±0.092* | 0.003±0.060# |
| Scleral cup depth (mm) | −0.362±0.131* | −0.208±0.095*# | 0.021±0.280 | 0.552±0.103*# |
| Optical axial length (mm) | −0.493±0.115* | −0.379±0.106* | -0.180±0.369 | 0.746±0.166*# |
Interocular difference* or difference between CL & NL groups# statistically significant, p<0.05.
Apart from the differences just noted between the CL and NL groups wearing +10 D lenses, there are other subtle differences in their response patterns that may reflect an interaction between the effects of CL and plus lenses. First, only the CL group showed a relative reduction in anterior chamber depth in the lens-wearing eyes (Table 2), and the difference between the CL and NL groups was also significant for this effect. Second, while most of the choroidal thickening occurred over the first 2 days for both groups (0.306 ± 0.059 mm, CL, p<0.0001; 0.206 ± 0.064 mm, NL, p=0.0005), this thickening response was more sustained in the NL group. Thus choroidal thickness changes remained statistically significant for the NL group at the end of the lens-wearing period although they were not significant for the CL group. Nonetheless, both groups showed significant regression of the choroidal thickness changes over the latter half of this period (−0.312 ± 0.075 mm, CL, p<0.0001; −0.112 ± 0.066 mm, NL, p<0.01). This regression of the choroidal thickening responses presumably reflects the contribution to compensation of the sclera that underwent a delayed inhibition as mirrored in the relative decrease in scleral cup lengths (Figure 1b, left); for the same reason, no regression of the VC response is evident.
Minus lensess
With the –10 D lens, only the early response of the CL group was in the correct direction for compensation; the overall response pattern for this group was in the wrong direction. Under NL, VC and OL elongation was enhanced and the resulting myopic shift in refraction almost completely neutralized the imposed hyperopia (Figure 1; Table 2). This response profile was seen across all chicks (Figure 2, right panel) and is also consistent with previously reported findings (Wildsoet 2003). In contrast, while the majority of eyes in the CL group also showed an early myopic shift in refractive error (−1.5 ± 2.0 D, CL, p<0.05, vs. −3.0 ± 1.0 D, NL, p<0.005; day 9; Figure 2, left panel), these changes did not prevail. Overall the lenses induced a slight increase in hyperopia, with 8 out of 12 of lens-treated eyes showing increased hyperopia (Figure 2, left panel), instead of increased myopia, like the NL group. Consistent with these changes in refractive errors, the eyes of the CL group showed early increases in VC depth (0.105 ± 0.15 mm, CL, p<0.05, vs. 0.105 ± 0.067 mm, NL, p<0.05; day 9) (Figure 1, right), with the majority of eyes showing later decreases. However the early increases in OL did not reach statistical significance for the CL group (0.046 ± 0.15 mm, CL, NS, vs. 0.136 ± 0.07 mm, NL, p<0.05; day 9). Thus there was minimal change in VC depth and OL overall (Figure 1, right & Figure 2, left panels). There are also other differences between these NL and CL groups; for the NL group, lens wear had little effect on CT and CR but resulted in a relative increase in AC growth while the CL group recorded minimal change in AC depth and small increases in CT and CR. Intergroup differences attained statistical significance for RE, CR, AC depth, LT, VC depth, CT and OL (Table 2).
Figure 2.
The changes in interocular differences (treated eyes - fellow eyes) in ocular parameters over the lens-wearing period for individual chicks wearing minus lenses: a) refractive error (RE), b) optical axial length (OL). Dashed lines represent chicks in constant light (CL), left panel. Solid lines represent chicks in normal lighting cycle (NL), right panel. Thick lines represent group mean data (NL: half-filled squares, CL: circles), error bars denote SEs. Pre-normalized data after further normalization to day 7 values plotted. All of the NL group compensated for the imposed defocus, developing substantial myopia and increased OLs. While the majority of the CL group also responded initially in the same (correct) direction, 8 out of the 12 eyes developed increased hyperopia and shorter OLs, these changes being in the wrong direction for compensation.
CL-lens treatment effects compared
Although the overall response patterns of the lens-treated eyes of the two CL groups shared some features, the hyperopia resulting from the late change in response direction in the −10 D lens group never achieved levels comparable to the values elicited by the +10 D lens, and statistical comparison of these two groups yielded significant results for most parameters (RE, p<0.0001; CR, p<0.01; AC depth, p<0.0001; LT, p<0.0005; VC depth, p<0.005; OL, p<0.0001).
Recovery from lens-induced changes under CL or NL conditions
All eyes were able to recover from lens-induced changes on removal of the lenses, irrespective of the previous lens treatment and lighting conditions during the recovery period.
The three plus lens groups, i.e. NLrNL, CLrCL and CLrNL groups, all entered the recovery period with shorter than normal VC depths and OLs and large hyperopic errors (Figure 3, left), and all showed significant overall relative myopic shifts in RE, and increases in LT, VC depth and OL, compared to the changes in the fellow untreated eyes during the recovery period (Table 3).
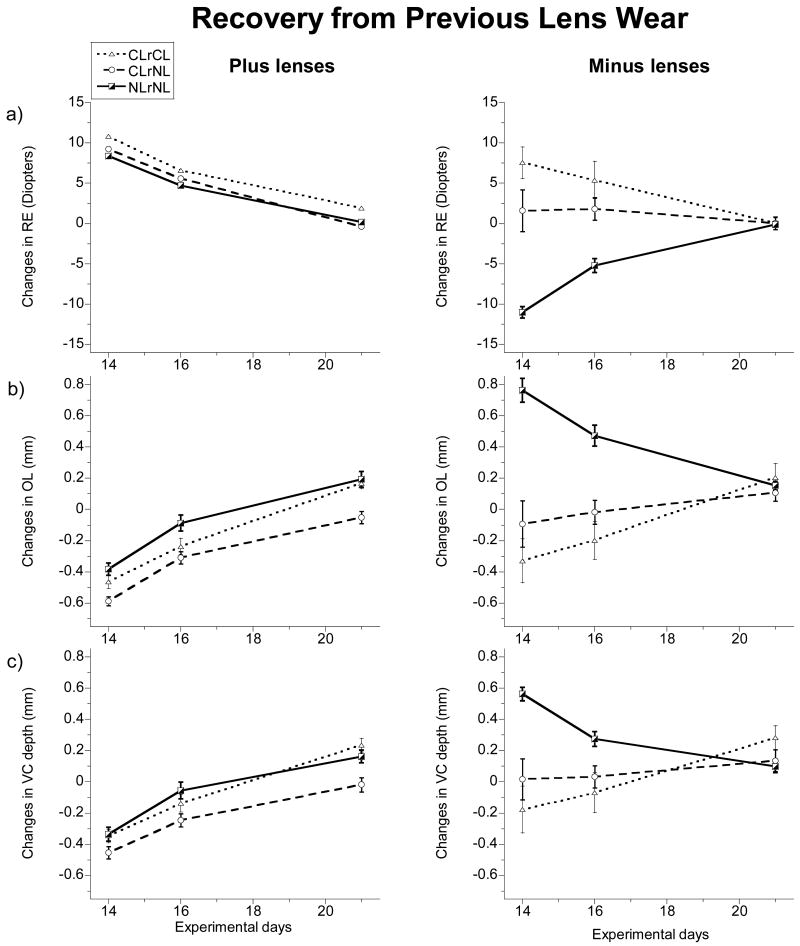
Figure 3.
The interocular differences (treated eyes - fellow eyes) in ocular parameters (a) refractive error (RE), b) optical axial length (OL), c) vitreous chamber (VC) depth over the recovery period in previous lens-wearing chicks (left, plus lens; right, minus lens) Means ± SE for pre-normalized data are plotted. Dashed lines with circles represent chicks reared in CL throughout; CLrCL). Dotted lines with triangles indicate CLrNL chicks (chicks reared in CL followed by NL). Solid lines with half-shaded squares represent data from chicks reared in NL throughout; NLrNL). Treated eyes that had worn plus lenses were relatively hyperopic and shorter in OLs and VCs than their respective fellow eyes and over the recovery period, these eyes showed relative decreases in hyperopia with increased elongation of OLs and VCs. Eyes made myopic with minus lenses (NLrNL group) initially had longer VCs and OLs than their fellow eyes and showed relative decreases in myopia with slowed growth in VCs and OLs. The previously minus lens-treated eyes of the CLrCL group were similar to their fellows at the start of the recovery period and remained so, while those of the CLrNL group, which were slightly hyperopic initially, showed decreases in hyperopia, with increased elongation of their VCs and OLs.
Table 3.
Mean interocular differences (± SD) in the changes in ocular parameters over the week following removal of the lenses, when the lighting conditions during the lens-wearing were left unchanged (CLrCL and NLrNL) or the CL conditions replaced by NL conditions during the recovery period (CLrNL). Raw data were initially normalized to day zero (pre-normalized) and then further normalized to day 14 readings to isolate the effects of the lens treatments.
| Ocular parameter | +10 D lens | −10 D lens | ||||
|---|---|---|---|---|---|---|
| CLrCL | CLrNL | NLrNL | CLrCL | CLrNL | NLrNL | |
| Refractive error (D) | −9.0±2.0* | −9.5±1.25* | −8.25±0.5* | −1.5±1.75# | −7.5±1.0*#‡ | +11.0±1.25* |
| Corneal radius of curvature (mm) | −0.025±0.106* | 0.011±0.121* | −0.069±0.082* | −0.067±0.114*# | 0.002±0.039* | 0.081±0.071* |
| Anterior chamber depth (mm) | 0.024±0.095 | −0.015±0.076 | 0.003±0.033 | 0.012±0.111 | −0.023±0.053 | −0.089±0.085 |
| Lens thickness (mm) | 0.078±0.033* | 0.068±0.02* | 0.073±0.046* | 0.069±0.027*# | 0.096±0.030*# | −0.058±0.046* |
| Vitreous chamber depth (mm) | 0.435±0.119* | 0.580±0.104*# | 0.499±0.102* | 0.119±0.186# | 0.46±0.169*#‡ | −0.463±0.089* |
| Choroidal thickness (mm) | −0.041±0.035* | −0.054±0.106 | −0.094±0.060* | −0.081±0.052*# | −0.224±0.142*#‡ | 0.169±0.113* |
| Optical axial length (mm) | 0.537±0.096* | 0.633±0.076* | 0.575±0.100* | 0.199±0.130*# | 0.534±0.174*#‡ | −0.611±0.059* |
Interocular difference* or difference from NLrNL group# and CLrCL group‡ statistically significant, p<0.05.
The 3 minus lens groups (NLrNL, CLrCL & CLrNL groups), also showed recovery responses although the already described differences in profiles of the NLrNL group compared to the CLrNL and CLrCL groups at the start of the recovery period resulted in differences in their recovery patterns. Indeed, their recovery responses are opposite in direction to each other (Figure 3, right); for NLrNL group, there was a relative hyperopic shift in refraction, decrease in VC depth and an increase in CT that contributed to a decrease in OL. These eyes also had consistently deeper AC depths relative to those of their fellows (0.146 ± 0.085 mm, p<0.05), offering an explanation for why the choroids of these eyes remained expanded at the end of the recovery period (0.178 ± 0.113 mm, p<0.05). By random chance, the previously lens-treated eyes of the CLrCL group were not different from their fellow eyes at the start of the recovery period, except for having thinner than normal lenses, and predictably, these eyes recorded a significant greater change in LT compared to their fellows over this period (0.069 ± 0.027 mm, p=0.0005). These eyes also showed a significant decrease in CT (−0.081 ± 0.052 mm, p<0.01) and increase in OL (0.199 ± 0.130 mm, p<0.01) relative to their fellow eyes over this period. On the other hand, the treated eyes of the CLrNL group, which were more hyperopic with thinner lenses and flatter corneas than those of their fellow eyes at the start of the recovery period (RE: +7.5 ± 4.75 D, p<0.05; LT: −0.108 ± 0.025 mm, p<0.001; CR: 0.099 ± 0.068 mm, p<0.05), exhibited a significant reduction in hyperopia associated with relative increases in LT, CR, VC depth, CT and OL over the recovery period (Table 3).
By the end of the recovery period, all groups had almost completely recovered from lens-induced changes, irrespective of the sign of lens worn previously (Figure 3a). Thus the ocular dimensions of treated and fellow eyes were closely matched at this time for both the plus and minus lens-CLrCL groups, except for slightly greater hyperopia in treated eyes relative to their fellow eyes in the +10 D lens-CLrCL group (+2.0 ± 2.0 D, p<0.05). Some interocular differences also were noted in the CLrNL and NLrNL groups. For example, the VCs of treated eyes of both of the CLrNL groups and the plus lens-NLrNL group were significantly longer than those of their respective fellows at the end of the recovery period (0.234 ± 0.104 mm, plus-CLrNL, p<0.005; 0.282 ± 0.169 mm, minus-CLrNL, p<0.05; 0.163 ± 0.102 mm, plus-NLrNL, p<0.05) (Figure 3c), and similar trends also were evident in the OL data for the plus lens-CLrNL group and both NLrNL groups (0.168 ± 0.076 mm, plus-CLrNL, p<0.005; 0.193 ± 0.1 mm, plus-NLrNL, p<0.01; 0.153 ± 0.059 mm, minus-NLrNL, p<0.005) (Figure 3b).
CL-induced changes & recovery in NL
Many of the now well-characterized effects of CL on eye growth in the chick (Jensen and Matson 1957; Kinnear, Lauber et al. 1974; Li, Troilo et al. 1995) were evident after only 7 days of CL (both eyes of chicks reared in CL), but became more exaggerated after a further 14 days in CL (untreated fellow eyes of the CLrCL group). AC growth was affected early, with a shallowing of AC depth evident in the data by day 7 (Tables 4 & 5). This difference in AC growth between the NL and CL groups also was statistically significant (−0.108 ± 0.052 mm, p<0.0001, day 7) (Figure 4a). VCs increased more than normal in CL but this effect of CL developed more slowly than the AC effect, with changes not reaching statistical significance until the end of the third week (0.253 ± 0.299, p<0.05, day 21, CLrCL group) (Figure 4b). Small, albeit statistically significant, decreases in CT were recorded at the end of the second week in CL (CT: −0.050 ± 0.038 mm, p<0.001; data from CLrCL & CLrNL groups pooled). CL did not significantly alter RE (Table 5).
Table 4.
Mean ocular parameters (± SD) on day 7, at the end of the pre-lens period, for eyes subsequently fitted with lenses (data not normalized), for chicks reared in constant (CL) and normal (NL) lighting. Both the unpooled & pooled data across lens treatment groups are presented.
| Ocular parameter | Constant lighting | Normal lighting | ||||
|---|---|---|---|---|---|---|
| +10 D lens group | −10 D lens group | Pooled data | +10 D lens group | −10 D lens group | Pooled data | |
| Refractive error (D) | +2.5±1.25 | +3.0±1.5 | +2.75±1.5 | +3.25±1.25 | +2.75±1.0 | +3.25±1.0 |
| Corneal radius of curvature (mm) | 3.240±0.139 | 3.203±0.108 | 3.222±0.124 | 3.160±0.099 | 3.326±0.154 | 3.235±0.148 |
| Anterior chamber depth (mm) | 1.347±0.052 | 1.328±0.032 | 1.338±0.044 | 1.445±0.025 | 1.474±0.038 | 1.458±0.034 |
| Lens thickness (mm) | 2.139±0.061 | 2.157±0.049 | 2.148±0.055 | 2.136±0.062 | 2.149±0.035 | 2.142±0.049 |
| Vitreous chamber depth (mm) | 5.535±0.265 | 5.446±0.193 | 5.492±0.233 | 5.354±0.191 | 5.587±0.080 | 5.460±0.189 |
| Choroidal thickness (mm) | 0.192±0.035 | 0.175±0.041 | 0.184±0.038 | 0.230±0.037 | 0.208±0.040 | 0.220±0.038 |
| Optical axial length (mm) | 9.021±0.297 | 8.932±0.208 | 8.978±0.257 | 8.935±0.224 | 9.210±0.108 | 9.060±0.224 |
Table 5.
Mean ocular parameters (± SD) of the untreated fellow eyes recorded on days 7, 14 and 21 of the 3-week study period; either CL or NL conditions prevailed (CLrCL and NLrNL) or CL conditions during the lens-wearing period were replaced by NL conditions during recovery period (CLrNL). Data were not normalized.
| Ocular parameter | Day | CLrCL | CLrNL | NLrNL |
|---|---|---|---|---|
| Refractive error (D) | 7
14 21 |
+3.25±1.0
+2.75±0.75 +2.25±0.75 |
+2.5±1.25
+3.0±0.5 +2.25±0.75 |
+3.0±1.0
+3.25±0.75 +2.5±0.75 |
| Corneal radius of curvature (mm) | 7
14 21 |
3.217±0.117
3.549±0.091 3.862±0.131 |
3.279±0.128
3.647±0.140 3.837±0.153 |
3.262±0.134
3.541±0.106 3.782±0.070 |
| Anterior chamber depth (mm) | 7
14 21 |
1.338±0.053
1.317±0.085 1.350±0.119 |
1.334±0.051
1.310±0.088 1.632±0.115 |
1.448±0.047
1.589±0.042 1.707±0.044 |
| Lens thickness (mm) | 7
14 21 |
2.162±0.053
2.400±0.054 2.582±0.064 |
2.149±0.053
2.381±0.058 2.560±0.050 |
2.142±0.053
2.348±0.056 2.539±0.064 |
| Vitreous chamber depth (mm) | 7
14 21 |
5.465±0.235
5.960±0.250 6.416±0.299 |
5.608±0.169
6.092±0.262 6.270±0.249 |
5.493±0.210
5.859±0.197 6.201±0.223 |
| Choroidal thickness (mm) | 7
14 21 |
0.170±0.023
0.170±0.037 0.180±0.041 |
0.173±0.036
0.183±0.039 0.411±0.060 |
0.200±0.040
0.232±0.031 0.210±0.022 |
| Optical axial length (mm) | 7
14 21 |
8.965±0.272
9.677±0.278 10.348±0.311 |
9.090±0.182
9.783±0.228 10.461±0.231 |
9.083±0.253
9.795±0.237 10.447±0.276 |
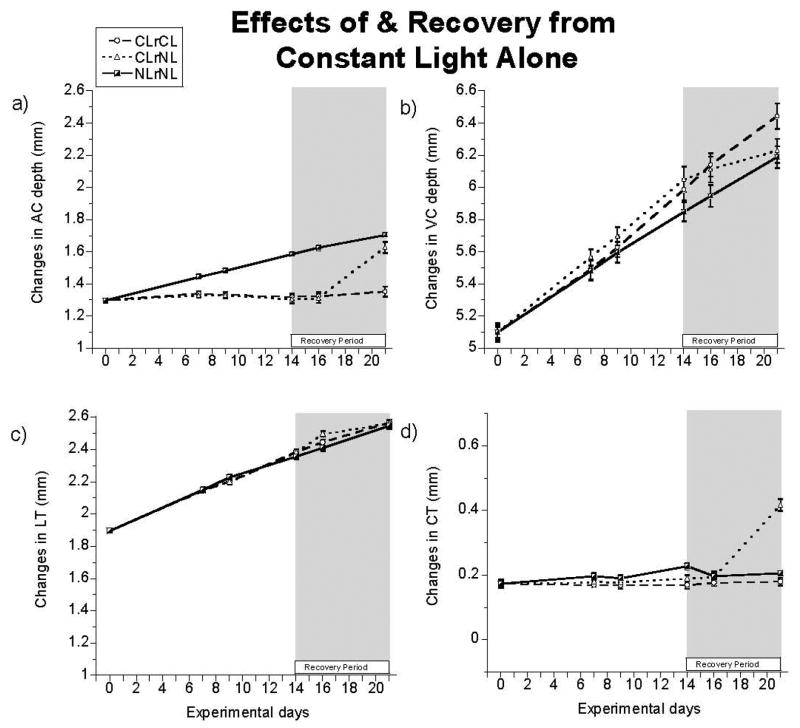
Figure 4.
The changes in ocular parameters over the experimental period in the untreated eyes: a) vitreous chamber (VC) depth, b) anterior chamber (AC) depth, c) lens thickness (LT) and d) choroidal thickness (CT). Means ± SE for pre-normalized data plotted. Dashed lines with circles represent chicks reared in CL throughout (CLrCL). Dotted lines with triangles indicate chicks reared in CL followed by NL (CLrNL). Solid lines with half-shaded squares represent chicks reared in NL throughout (NLrNL). CL rearing slowed AC growth while increasing VC growth. On return to NL, eyes recovered completely; compare VC and AC depths of CLrNL and NLrNL groups. A delayed but sustained increase in CT contributed to the regression in CL-induced increase in VC depth in the CLrNL eyes. The recovery response of the AC was also delayed, for 2 days of transfer to NL. An early transient increase in LT upon returning to NL was also evident.
Just one week in NL was sufficient to reverse the CL-induced growth patterns. Thus, AC growth accelerated while VC growth slowed in the fellow untreated eyes of the CLrNL group, resulting in significant differences in growth from the other 2 groups (AC depth: CLrNL vs. other two groups, p<0.0001; VC depth: CLrNL vs. CLrCL, p<0.05). In both cases, the changes showed a delayed onset, reflecting in the case of VC growth, a similar delay in choroidal thickening that contributed to the recovery response (CLrNL vs. other two groups, p<0.0001) (Figure 4d). By day 21, the fellow eyes of the CLrNL group had similar AC and VC depths, as well as LTs to those of the NLrNL group although LT showed an early transient increase (CLrNL vs. other two groups, p<0.05, day 16) (Figure 4c), and CTs and RTs remained thicker on day 21 (CT: CLrNL vs. other two groups, p<0.0001; RT: CLrNL vs. other two groups, p<0.05). Although no statistically significant change in CR was observed under CL, a small but significant decrease in CR was recorded over the recovery period (CLrNL vs. CLrCL, p<0.05). There were no significant intergroup differences in RE over the recovery period (Table 5).
Discussion
The three main findings of this chick-based study are that: 1) compensation to the defocus imposed by minus lenses is disrupted by CL conditions, while compensation for the defocus imposed by plus lenses is near normal, albeit slightly exaggerated; 2) eyes can recover in both CL and NL from experimentally-induced hyperopia when the inducing stimulus is removed; 3) eyes can recover from CL-induced alterations in their ocular dimensions when shifted to NL. The following discussion will consider these data in the context of the mechanisms underlying ocular growth regulation and emmetropization.
Responses to lens-induced defocus in CL compared to NL conditions
Under NL conditions, chicks respond in a very predictable and reliable way to imposed defocus (Irving, Callender et al. 1995; Wildsoet and Wallman 1995), elongating faster than normal in the presence of minus lenses and slowing their growth in response to plus lenses. Both the sclera and choroid contribute to these responses, the net refractive effect of minus and plus lenses being induced myopia and hyperopia respectively, reflecting the associated changes in vitreous chamber depth. Choroidal responses take the form of thinning with imposed hyperopia, and thickening with imposed myopia, and tend to precede scleral responses that are expressed as changes in scleral thickness and/or the length of the scleral cup.
Equivalent data collected under CL show a similar, albeit slightly exaggerated, response profile for plus lenses but eyes failed to compensate for the defocus imposed by the minus lenses in CL. Nonetheless, both plus and minus lenses elicited responses, implying that the defocus stimulus was detected in both cases. While the earlier study by Bartmann and co-workers (1994) using a bilateral lens paradigm reported near normal responses to imposed defocus under CL, visual inspection of the graphs included in this paper suggests that the plus lens response is exaggerated, while the minus lens response is inhibited, just as we found. A lack of an adaptation period and/or the use of hatchling chicks in the only other related study, by Guo and co-workers (1996), may underlie the different outcomes of our two studies – partial compensation versus a failure of compensation to imposed hyperopia in the current study. For example, hatchlings have functionally immature retinas (Schmid and Wildsoet 1996b; Fosser, Brusco et al. 2005) and it is likely to take at least a couple of days of exposure to CL for ocular rhythms to be lost (Weiss and Schaeffel 1993) (Parkinson and Rando 1983; Zawilska, Bednarek et al. 2003).
Taking into account that CL alone enhances VC elongation, the failure of eyes wearing minus lenses in CL to further increase their rate of growth to compensate for the imposed hyperopia, as observed in the current study, could indicate a limited capacity of eyes to upregulate their growth. This “maximum-growth” model can explain the results of Guo et al. (1996) who reported partial compensation to minus lenses after 2 weeks in CL and a similar explanation has been offered for the altered responses of eyes to form deprivation in CL (Bartmann, Schaeffel et al. 1994). However, while the majority of eyes (67%), initially grew in the correct direction for compensation to minus lenses in CL in the current study, only 25% of eyes maintained this growth pattern over the remainder of the treatment period. This reversal in the direction of growth contrasts with the effect of increasing the amount of imposed hyperopia in NL, which is to increase the time required for compensation rather than a reversal of growth direction (Wildsoet 2003; Yew 2004). Thus our CL data suggests an error in the decoding of the sign of the imposed defocus.
An alternative explanation for the failure of eyes to compensate to the minus lenses in CL that need not preclude a decoding error as raised above, focuses on the role of retinal dopamine in eye growth regulation (Retinal dopamine theory). Indeed, the dopamine (DA) depleting effect of CL (Lauber 1987; Morgan, Ashby et al. 2004) offers a potential explanation for why both form deprivation and minus lens responses are impaired in CL. For example, form deprivation is known to alter retinal DA rhythms, and also reduce turnover (Stone, Lin et al. 1989); if these changes are causally linked to the induced growth response, then one would predict an attenuated response to form deprivation under conditions that prematurely deplete retinal DA. Also of interest to the current study is the finding that short-term CL exposure (2 days) increases the daytime retinal DA levels, at the same time suppressing the DA rhythm (Parkinson and Rando 1983; Zawilska, Bednarek et al. 2003), contrasting with the long-term DA lowering effect of CL. This difference between the short and longer term effects of CL on retinal DA could explain the partial responses to minus lenses reported in the Guo et al (1996) study in which the lenses were added at the start of the CL period. These arguments in relation to minus lens responses rest on the assumption that they also deplete retinal DA, like form deprivation. Also, if myopic defocus is signaled via an increase in retinal dopamine, this would explain why the plus lens responses were preserved. However, there is no consistency in the results from lens studies in chicks in relation to their effects on retinal DA (Bartmann, Schaeffel et al. 1994; Guo, Sivak et al. 1995; Schaeffel, Bartmann et al. 1995; Ohngemach, Hagel et al. 1997; Boelen, Megaw et al. 1999). Further investigations into the effects of lens wear in CL on retinal dopamine release and metabolism are warranted to follow-up on these conjectures.
Our finding that altered light exposure during rearing selectively disrupts compensation to minus lenses in chicks is not new in of itself. Kee et al. (2001) found that interrupting the dark (night) cycle with brief periods of light also disrupted compensation to minus lenses but did not impair compensation to plus lenses. Because these conditions, like CL, are expected to perturb diurnal rhythms, it is tempting to argue, based on our data and that of Kee et al., that a normal diurnal light cycle is an essential pre-requisite for compensation to minus lenses. However, the finding of Yew et al. (2003), that such interrupted night effects are also lens power-dependent suggests that this interpretation is an over-simplification.
The main focus of the above discussion has been on the response to minus lenses in CL. With the experimental paradigm used in the current study, the response to plus lenses was slightly exaggerated; eyes in CL initially showed greater choroidal thickening and their scleral cups elongated less compared to eyes in NL. These results are interesting in that they indicate that the growth signals generated by plus lenses are more robust than those generated by the CL conditions which promote scleral growth and choroidal thinning. In this respect, there is a parallel between the responses to CL and minus lenses (hyperopic defocus), both of which fail in competition with plus lenses (myopic defocus) (Winawer and Wallman 2002; Diether and Wildsoet 2005). Also, while eyes compensate for repeated brief exposure to plus lenses, they do not compensate for equivalent periods of minus lens wear (Winawer and Wallman, (2002). These direction-related differences in ocular growth responses may reflect related differences in their temporal dynamics or the nature of the responses. Nonetheless, it is unclear why Guo et al. observed only partial compensation to plus lenses when we found this response to be slightly exaggerated, although inter-study differences in the age of the chicks may be a contributing factor.
Evidence for different mechanisms underlying compensation to lens-imposed hyperopia & experimentally-induced hyperopia
That chicks reared in NL were able to recover from lens-induced changes when the lenses were removed, irrespective of the sign of the imposed defocus, is consistent with earlier reports (Wildsoet and Wallman 1995), and so expected. However, that chicks reared in CL could recover from induced hyperopia, under both CL and NL conditions is counterintuitive, given that eyes showed only transient, partial compensation for hyperopic defocus when imposed by minus lenses in CL. These disparate findings argue against the notion of a shared mechanism for lens compensation and recovery responses, at least for hyperopic defocus.
In the current study, the changes during the recovery period mostly reversed those induced over the lens-wearing period. Thus in the CLrCL group, eyes that became hyperopic in response to lens wear, subsequently showed relatively greater increases in vitreous chamber depth and optical axial lengths than their untreated fellow eyes, to become similar to them in terms of ocular dimensions, although in terms of refractions, they were relatively hyperopic. While others have raised the possibility of passive shape mechanisms in explaining the recovery of normal ocular shape in eyes made to grow away from their normal dimensions through experimental intervention (Schaeffel and Howland 1991; Troilo and Wallman 1991), it must be noted that the CL conditions experienced by the CLrCL group preclude eyes ever achieving normal ocular dimensions. Thus recovery from induced hyperopia under CL is likely to be at least partly visually-guided, a conclusion that seems to apply to recovery data generally, based on the results of another study showing that optical correction of induced myopia inhibits recovery (Wildsoet and Schmid 2000). Analyses of growth rates during the recovery process in the current study are also more consistent with active emmetropization, at least under NL conditions. Specifically, growth rates of both the vitreous chamber and optical axial length increased to become greater than normal in eyes responding to induced hyperopia but slower below normal for induced myopia. Further implicating active processes is the finding that eyes had longer vitreous chambers and optical axial lengths compared to their fellow eyes, after recovering from induced hyperopia in NL, irrespective of the previous lighting conditions. Only those recovering in CL became closely matched in ocular dimensions to their fellow eyes. Thus visually-guided mechanisms must be acting in concert with any passive, non-visual mechanism to allow young eyes to recover from induced refractive errors.
The recovery of previously lens-treated eyes of chicks switched from CL to NL indicates that any effect of CL on emmetropization is reversible on restoration of normal lighting. These eyes provide further evidence of a contribution of active emmetropization to such recovery responses, their vitreous chambers showing greater elongation than their fellow eyes whose vitreous chambers also normalized in dimensions with the switch from CL to NL conditions. They also provide evidence for differential regulation of the anterior and posterior vitreous chamber segments as described elsewhere (Wildsoet and Pettigrew 1988a; Wildsoet and Wallman 1995; Fischer, Morgan et al. 1999). Specifically, while there were interocular differences in vitreous chamber elongation, increases in anterior chamber depth were similar for treated eyes and their fellows. Finally, that intraocular pressure (IOP) was lower in the eyes of chicks reared in CL (as reported by Li et al., 2002), and recovered to normal levels in those returned to NL (Padmanabhan 2005), suggests a possible interdependence between intraocular pressure (IOP) and anterior segment development.
Why do eyes retain their ability to recover from induced hyperopia yet lose their ability to compensate for the hyperopia imposed by minus lenses in CL? While both recovery from induced hyperopia and compensation to lens-imposed hyperopia require similar directions of growth, the starting points are different; specifically, for hyperopia to be induced by the lenses, eye growth had to be slowed and with the removal of the lenses during the recovery, this restraining influence on now relatively shorter eyes was eliminated. As anterior segment changes also had largely stabilized, even a return to “normal” growth (CL-induced increases vitreous chamber elongation), would have facilitated recovery. The influences of ocular growth rhythms in these recovery processes are less clear. Thus while a study by Nickla et al. (2006) links ocular growth rates with the phase relationship of the rhythms in axial elongation and choroidal thickness, for eyes of chicks responding to lens-imposed hyperopia (minus lens response) and eyes recovering from induced hyperopia, our result that CL does not perturb the recovery response to induced hyperopia although it is known to perturb ocular growth rhythms on its own, suggests a more complex picture.
Choroidal contribution to recovery from CL-induced changes
Although recovery from CL-induced ocular changes has been reported previously (Li, Wahl et al. 2002; Li, Wahl et al. 2004), our study represents the most comprehensive study of its kind, with high frequency A-scan ultrasonography providing new insight into the changes in the posterior layers of the eyes. In the current study, the effects of CL had largely been reversed after a week in NL with the exception of choroidal thickness that progressively thickened over this period. The latter response is likely to be compensatory, as the normalization of anterior chamber depth dimensions would have resulted in an increase in the optical axial length and myopia over the same period, had these changes not been offset by choroidal thickness changes. This result also implies that active emmetropization was once again functioning, even if perturbed under the CL conditions. In the current study, there were no significant changes in either corneal radius of curvature or refractive error in eyes exposed to the 3 weeks of CL, although slight corneal steepening was observed in eyes returned to NL for the last week of the study period. These results contrast with the observations of hyperopia and corneal flattening in just 2 weeks of CL in the Cornell-K strain of White-Leghorn chicks (Li, Troilo et al. 1995; Stone, Lin et al. 1995). And recovery also appears to be slower in this strain although the direction of the changes are similar to those reported here (Wahl, Li et al. 2002). These differences are likely to be breed-related as reported previously (Stone, Lin et al. 1995; Troilo, Li et al. 1995; Schmid and Wildsoet 1996a).
Are there parallels between the effects of CL in chicks in monkeys & humans
As already referred to in the introduction, a number of studies have explored the possible link between the duration of daily light exposure and myopia in humans (Quinn, Shin et al. 1999) (Chapell, Sullivan et al. 2001; Loman, Quinn et al. 2002; Czepita, Goslawski et al. 2004). Because of confounding factors in these studies and negative findings in other related studies (Gwiazda, Ong et al. 2000; Zadnik, Jones et al. 2000; Saw, Wu et al. 2001; Saw, Zhang et al. 2002; Guggenheim, Hill et al. 2003), a causal link between excessive light exposure and myopia in humans remains to be established. Even if proven, there is likely to be a critical period within which light-induced changes are reversible, based on the findings reported here.
In studies investigating the effects of CL on emmetropization in the rhesus monkey, neither lens compensation or recovery responses in monkeys were found to be compromised by CL (Smith, Hung et al. 2003), and otherwise untreated eyes show normal growth patterns in CL (Smith, Bradley et al. 2001; Smith, Hung et al. 2003). Although the possibility that monkeys cover their eyes while sleeping in CL cannot be ruled out as an explanation for this negative result, there is evidence for a role of pineal gland in the CL effects on eye growth in the chick (Li and Howland 2003), with no equivalent pineal gland-mediated mechanism being plausible in monkeys because of its deeper location [this is true of primates as well as mammals (Lockley, Skene et al. 1998; Meijer, Thio et al. 1999; Skene, Lockley et al. 1999; Yamazaki, Goto et al. 1999)], protection from light exposure by a much thicker cranium (Smith, Bradley et al. 2001; Smith, Hung et al. 2003). However, there are also local ocular growth regulatory mechanisms that are perturbed by CL, at least in chick (Li and Howland 2003). Further investigations are required to understand more about these ocular circuits and whether equivalent circuits exist in the primate eye.
Conclusion
In the current study, rearing chicks in constant light was found to differentially affect the compensatory responses to imposed focusing errors, disrupting the response to hyperopia (minus lenses), with minimal effect on the response to myopia (plus lenses). Paradoxically, these rearing conditions had little effect on the ability of eyes to recover from induced hyperopia, the product of lens wear. These results imply that different mechanisms underlie the responses to these two types of hyperopia. It is possible that the altered response to minus lenses in CL is linked to the retinal dopamine-depleting effect of CL, although eyes that are already elongating faster than normal, as in CL, may be limited in their capacity further increase their rate of vitreous chamber elongation to compensate for imposed hyperopia. Because eyes with induced hyperopia are initially shorter than their fellows, even a return to normal growth will facilitate recovery. The effects of CL were reversible, as evidenced by the ability of eyes to recover from both lens- and CL-induced refractive changes when animal are returned to normal light. Understanding more about the changes to the dopamine and ocular growth rhythms changes provoked by the visual and lighting manipulations used in this study could provide more insight into their roles in emmetropization.
Acknowledgments
This work was undertaken with the support of an NEI grant to CFW (EY12392).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Au Eong KG, Tay TH, et al. Education and myopia in 110,236 young Singaporean males. Singapore Med J. 1993;34(6):489–92. [PubMed] [Google Scholar]
- Bartmann M, Schaeffel F, et al. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Vis Neurosci. 1994;11(2):199–208. doi: 10.1017/s0952523800001565. [DOI] [PubMed] [Google Scholar]
- Boelen MK, Megaw P, et al. Retinal dopamine does not decode for the sign of defocus. Invest Ophthalmol Vis Sci. 1999;40(Suppl):963. [Google Scholar]
- Chapell M, Sullivan B, et al. Myopia and night-time lighting during sleep in children and adults. Percept Mot Skills. 2001;92(3 Pt 1):640–2. doi: 10.2466/pms.2001.92.3.640. [DOI] [PubMed] [Google Scholar]
- Curtin BJ. The myopias-basic science and clinical management. Philadelphia: Harper & Row; 1985. [Google Scholar]
- Czepita D, Goslawski W, et al. Role of light emitted by incandescent or fluorescent lamps in the development of myopia and astigmatism. Med Sci Monit. 2004;10(4):CR168–71. [PubMed] [Google Scholar]
- Diether S, Wildsoet CF. Stimulus requirements for the decoding of myopic and hyperopic defocus under single and competing defocus conditions in the chicken. Invest Ophthalmol Vis Sci. 2005;46(7):2242–52. doi: 10.1167/iovs.04-1200. [DOI] [PubMed] [Google Scholar]
- Edwards M. Is emmetropization a scale artifact? Optom Vis Sci. 1992;69(2):162–3. doi: 10.1097/00006324-199202000-00011. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, I, Morgan G, et al. Colchicine causes excessive ocular growth and myopia in chicks. Vision Research. 1999;39(4):685–697. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- Fosser NS, Brusco A, et al. Darkness induced neuroplastic changes in the serotoninergic system of the chick retina. Brain Res Dev Brain Res. 2005;160(2):211–8. doi: 10.1016/j.devbrainres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Guggenheim JA, Hill C, et al. Myopia, genetics, and ambient lighting at night in a UK sample. British Journal of Ophthalmology. 2003;87(5):580–582. doi: 10.1136/bjo.87.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SS, Sivak JG, et al. Retinal dopamine and lens-induced refractive errors in chicks. Curr Eye Res. 1995;14(5):385–389. doi: 10.3109/02713689508999936. [DOI] [PubMed] [Google Scholar]
- Guo SS, Sivak JG, et al. Effects of continuous light on experimental refractive errors in chicks. Ophthalmic Physiol Opt. 1996;16(6):486–490. [PubMed] [Google Scholar]
- Gwiazda J, Ong E, et al. Myopia and ambient night-time lighting. Nature. 2000;404(6774):144. doi: 10.1038/35004663. [DOI] [PubMed] [Google Scholar]
- Hofstetter HW. Emmetropization- biological process or mathematical artifact? Am J Optom Arch Am Acad Optom. 1969;46(6):447–50. [PubMed] [Google Scholar]
- Irving EL, Callender MG, et al. Inducing ametropias in hatchling chicks by defocus - aperture effects and cylindrical lenses. Vision Research. 1995;35(9):1165–1174. doi: 10.1016/0042-6989(94)00235-e. [DOI] [PubMed] [Google Scholar]
- Irving EL, Sivak JG, et al. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12(4):448–56. [PubMed] [Google Scholar]
- Jensen LS, Matson WE. Enlargement of avian eye by subjecting chicks to continuous incandescent illumination. Science. 1957;125(3251):741. doi: 10.1126/science.125.3251.741. [DOI] [PubMed] [Google Scholar]
- Kee CS, Marzani D, et al. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42(3):575–583. [PubMed] [Google Scholar]
- Kinnear A, Lauber JK, et al. Genesis of light-induced avian glaucoma. Invest Ophthalmol. 1974;13(11):872–875. [PubMed] [Google Scholar]
- Lauber JK. Light-induced avian glaucoma as an animal model for human primary glaucoma. J Ocul Pharmacol. 1987;3(1):77–100. doi: 10.1089/jop.1987.3.77. [DOI] [PubMed] [Google Scholar]
- Li T, Howland HC. The effects of constant and diurnal illumination of the pineal gland and the eyes on ocular growth in chicks. Invest Ophthalmol Vis Sci. 2003;44(8):3692–3697. doi: 10.1167/iovs.02-0990. [DOI] [PubMed] [Google Scholar]
- Li T, Troilo D, et al. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Research. 1995;35(9):1203–1209. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- Li T, Wahl C, et al. Intraocular pressure in chicks raised in constant light. Invest Ophthalmol Vis Sci. 2002;43 ARVO E-Abstract 197. [Google Scholar]
- Li T, Wahl C, et al. Age dependent ocular changes of chicks under constant light, and recovery from them, in normal illumination. Invest Ophthalmol Vis Sci. 2004;45 ARVO E-Abstract 4291. [Google Scholar]
- Lockley SW, Skene DJ, et al. Extraocular light exposure does not suppress plasma melatonin in humans. J Clin Endocrinol Metab. 1998;83(9):3369–72. doi: 10.1210/jcem.83.9.5244. [DOI] [PubMed] [Google Scholar]
- Loman J, Quinn GE, et al. Darkness and near work: myopia and its progression in third-year law students. Ophthalmology. 2002;109(5):1032–8. doi: 10.1016/s0161-6420(02)01012-6. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Thio B, et al. Functional absence of extraocular photoreception in hamster circadian rhythm entrainment. Brain Res. 1999;831(1–2):337–9. doi: 10.1016/s0006-8993(99)01509-7. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Ashby R, et al. The effect of constant light on the retinal dark-light switch and eye growth in the chicken. Invest Ophthalmol Vis Sci. 2004;45 ARVO E-Abstract 4292. [Google Scholar]
- Nevin ST, Schmid KL, et al. Sharp vision: a prerequisite for compensation to myopic defocus in the chick? Curr Eye Res. 1998;17(3):322–331. doi: 10.1076/ceyr.17.3.322.5220. [DOI] [PubMed] [Google Scholar]
- Nickla DL. The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192(4):399–407. doi: 10.1007/s00359-005-0077-2. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, et al. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res. 1997;16(4):320–6. doi: 10.1076/ceyr.16.4.320.10697. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, et al. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66(2):163–81. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- Ohngemach S, Hagel G, et al. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis Neurosci. 1997;14(3):493–505. doi: 10.1017/s0952523800012153. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V. MS Thesis, University of California, Berkeley. 2005. Mechanisms of emmetropization in chick- effect of continuous light and binocular interaction. [Google Scholar]
- Padmanabhan V, Wildsoet CF. Rearing in constant light disrupts compensation to negative lenses in chicks. Invest Ophthalmol Vis Sci. 2004;45 ARVO E-Abstract 4289. [Google Scholar]
- Parkinson D, Rando RR. Effects of light on dopamine metabolism in the chick retina. J Neurochem. 1983;40(1):39–46. doi: 10.1111/j.1471-4159.1983.tb12650.x. [DOI] [PubMed] [Google Scholar]
- Quinn GE, Shin CH, et al. Myopia and ambient lighting at night. Nature. 1999;399(6732):113–114. doi: 10.1038/20094. [DOI] [PubMed] [Google Scholar]
- Saw SM, Wu HM, et al. Myopia and night lighting in children in Singapore. British Journal of Ophthalmology. 2001;85(5):527–528. doi: 10.1136/bjo.85.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw SM, Zhang MZ, et al. Near-work activity, night-lights, and myopia in the Singapore-China study. Arch Ophthalmol. 2002;120(5):620–7. doi: 10.1001/archopht.120.5.620. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Bartmann M, et al. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Research. 1995;35(9):1247–1264. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, et al. Accommodation, refractive error and eye growth in chickens. Vision Research. 1988;28(5):639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Research. 1991;31(4):717–34. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- Schmid GF, Papastergiou GI, et al. Continuous light influences diurnal oscillation in axial length but not choroidal thickness in chicks. Invest Ophthalmol Vis Sci. 1997;38(Suppl):2529. [Google Scholar]
- Schmid K, Wildsoet C. Breed- and gender-dependent differences in eye growth and form deprivation responses in chick. J Comp Physiol [A] 1996a;178(4):551–561. doi: 10.1007/BF00190185. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Research. 1996b;36(7):1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Skene DJ, Lockley SW, et al. Effects of light on human circadian rhythms. Reproduction Nutrition Development. 1999;39(3):295–304. doi: 10.1051/rnd:19990302. [DOI] [PubMed] [Google Scholar]
- Smith EL, Bradley DV, et al. Continuous ambient lighting and eye growth in primates. Invest Ophthalmol Vis Sci. 2001;42(6):1146–1152. [PubMed] [Google Scholar]
- Smith EL, Hung LF, et al. Continuous ambient lighting and lens compensation in infant monkeys. Optom Vis Sci. 2003;80(5):374–382. doi: 10.1097/00006324-200305000-00012. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, et al. Photoperiod, early postnatal eye growth, and visual deprivation. Vision Research. 1995;35(9):1195–1202. doi: 10.1016/0042-6989(94)00232-b. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, et al. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989;86(2):704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Li T, et al. Differences in eye growth and the response to visual deprivation in different strains of chicken. Vision Research. 1995;35(9):1211–1216. doi: 10.1016/0042-6989(94)00230-j. [DOI] [PubMed] [Google Scholar]
- Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Research. 1991;31(7–8):1237. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- Wahl C, Li T, et al. Chick eyes recovering from constant light exposure have higher stromal cell counts and smaller venous sinuses but no change in stromal glycosaminoglycan content. Invest Ophthalmol Vis Sci. 2002;43 ARVO E-Abstract 209. [Google Scholar]
- Wallman J, Gottlieb MD, et al. Local retinal regions control local eye growth and myopia. Science. 1987;237(4810):73–7. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- Weiss S, Schaeffel F. Diurnal growth rhythms in the chicken eye: relation to myopia development and retinal dopamine levels. J Comp Physiol [A] 1993;172(3):263–70. doi: 10.1007/BF00216608. [DOI] [PubMed] [Google Scholar]
- Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks-insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res. 2003;27(6):371–85. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Research. 1995;35(9):1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF. Active emmetropization--evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17(4):279–90. [PubMed] [Google Scholar]
- Wildsoet CF, Pettigrew JD. Kainic acid-induced eye enlargement in chickens: differential effects on anterior and posterior segments. Invest Ophthalmol Vis Sci. 1988a;29(2):311–9. [PubMed] [Google Scholar]
- Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Research. 2000;40(23):3273–3282. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Research. 2002;42(24):2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Goto M, et al. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J Biol Rhythms. 1999;14(3):197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- Yew KL. MS Thesis, University of California, Berkeley. 2004. Is lens-induced myopia another type of form-deprivation myopia? Evidence from chicks using optical, lighting and surgical manipulations. [Google Scholar]
- Yew KL, Wildsoet CF. The usual effects of high-power negative lens and diffusers show different susceptibility to disruption to the diurnal light cycle. Invest Ophthalmol Vis Sci. 2003;44 ARVO E-Abstract 1979. [Google Scholar]
- Zadnik K, Jones LA, et al. Vision - myopia and ambient night-time lighting. Nature. 2000;404(6774):143–144. doi: 10.1038/35004661. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Bednarek A, et al. Rhythmic changes in metabolism of dopamine in the chick retina: the importance of light versus biological clock. J Neurochem. 2003;84(4):717–24. doi: 10.1046/j.1471-4159.2003.01559.x. [DOI] [PubMed] [Google Scholar]