Abstract
Modified vaccinia Ankara (MVA) is being tested in humans as an alternative to the current smallpox vaccine Dryvax. Here, we compare the magnitude and longevity of protective immune responses elicited by a DNA/MVA HIV-1 vaccine with those elicited by Dryvax using a monkeypox virus/macaque model. The DNA/MVA vaccine elicited similar levels of vaccinia virus (VV)-specific antibody and 5–10-fold lower levels of VV-specific cellular responses than Dryvax. This MVA-elicited cellular and humoral immunity was long-lived. A subset of the DNA/MVA- and Dryvax-vaccinated macaques were subjected to a lethal monkeypox virus challenge at 3 years after vaccination. All of the vaccinated monkeys survived, whereas the unvaccinated controls succumbed to monkeypox. The viral control correlated with early postchallenge levels of monkeypox-specific neutralizing antibody but not with VV-specific cellular immune response. Thus, our results demonstrate the elicitation of long lasting protective immunity for a lethal monkeypox challenge by a DNA/MVA HIV-1 vaccine.
Keywords: HIV, Modified vaccinia virus Ankara, DNA/MVA vaccine, Smallpox, Monkeypox, CD8 T cells, Rhesus macaque
Introduction
Although the World Health Organization certified the eradication of smallpox from the world in 1979, bioterrorist threats raise the possibility of the reintroduction of smallpox into a world, which is no longer being routinely vaccinated (Gani and Leach, 2001, Henderson, 1999a). There is a serious need for a new smallpox vaccine because of the incidence of adverse events to the current vaccine, Dryvax (Enserink, 2002). In 1968, 15 million people in the US were vaccinated with Dryvax. Of these, 240 required hospitalization, 9 died and 4 were permanently disabled (Henderson, 1999b). Also, many people are not qualified to receive Dryvax due to immunodeficiency from genetic causes, HIV, or immunosuppressive medicinal drugs, skin disorders (particularly eczema), old age, young age (< 1 year) or pregnancy. These groups represent a significant part of population and must be accounted for in any reasonable national smallpox vaccination strategy. Thus for vaccination to become routine in an unexposed population, smallpox vaccines with fewer side effects need to be developed (Weltzin et al., 2003).
The correlates for protection against smallpox likely involve both humoral and cellular immunity. A critical role for humoral immunity in protection is shown by resistance to smallpox exposure in individuals with high titers of vaccinia virus (VV)-specific neutralizing antibodies (Mack et al., 1972, Sarkar et al., 1975). Evidence for a critical role of cellular immunity comes from studies in individuals with T cell immunodeficiency. Such individuals are at high risk for developing disseminated vaccinia following smallpox vaccinations (O'Connell et al., 1964, Redfield et al., 1987). Studies in mice evaluating the mechanisms of vaccine-mediated protection against the pathogenic Western Reserve (WR) vaccinia virus strain have demonstrated critical protective roles for both VV-specific antibody (Belyakov et al., 2003, Wyatt et al., 2004a) and T cell responses (Wyatt et al., 2004a). Also studies with ectromelia virus demonstrate the importance of antibody (Panchanathan et al., 2006). These studies suggested a primary role for antibody and a secondary role for T cells in protection. Recently, studies in macaques have demonstrated an essential role for VV-specific antibody for protection against a monkeypox virus (MPXV) challenge using a macaque model of pathogenic poxvirus infection (Edghill-Smith et al., 2005b). In addition, CD4 T cells have been shown to be critical for generating protective antibody for MPXV in macaques with AIDS (Edghill-Smith et al., 2005a). However, none of the previous studies in non-human primates measured the poxvirus-specific T cell response following MPXV challenge limiting our understanding about the precise role of cellular immune response following challenge in the sub-human animal model of smallpox.
Up to the time of the eradication of smallpox, a number of efforts were made to develop a safer vaccine (Henderson, 1999b). One of these, further attenuation of a vaccine strain of vaccinia, resulted in the generation of modified vaccinia Ankara (MVA). MVA was attenuated for replication in humans by over 500 serial passages in chicken embryo fibroblast cells (Hirsch et al., 1996, Mayr et al., 1978). During these passages, MVA underwent six large genomic deletions (Antoine et al., 1998), which eliminated host range genes as well as immune evasion genes such as soluble receptors for IFN-γ, IFN α/β, tumor necrosis factor and CC-chemokines (Blanchard et al., 1998). The block in the replication of MVA in human cells occurs after the expression of the viral late genes but before virion morphogenesis (Sutter and Moss, 1992). The unimpaired late as well as early viral protein synthesis, even in non-permissive cells, coupled with the deletion of immune evasion genes, likely contributes to the usefulness of MVA as a safe yet efficient vaccine. Towards the end of the campaign for the eradication of smallpox, MVA was safely used in 120,000 humans (Stickl et al., 1974). However, by this time, the incidence of smallpox was sufficiently low that efficacy could not be determined.
There are no comparative studies in humans on VV-specific immunity raised by Dryvax and MVA. Studies in mice have compared VV-specific immune responses raised by the Western Reserve (WR) strain of vaccinia virus with those raised by MVA (Belyakov et al., 2003, Ramirez et al., 2000, Wyatt et al., 2004a). These studies demonstrated that the WR virus and MVA raise similar levels of anti-vaccinia antibody and T cells. Similarly, recent studies in cynomolgus macaques demonstrated that two inoculations of MVA elicit similar levels of anti-vaccinia antibodies as a single inoculation of Dryvax and confer protection from a lethal MPXV challenge that was administered by intravenous or respiratory route (Earl et al., 2004, Stittelaar et al., 2005). The protection conferred by MVA was similar to that conferred by Dryvax. These results strongly suggest that MVA could be a viable alternative to Dryvax.
MVA is also a popular vaccine vector for the expression of recombinant proteins (Amara et al., 2002b, Barouch et al., 2001, Earl et al., 2002, Hirsch et al., 1996, Sutter et al., 1994, Wyatt et al., 1996). We recently demonstrated that an AIDS vaccine consisting of a DNA prime and recombinant MVA boost (DNA/MVA SHIV vaccine) raises high titer simian and human immunodeficiency virus (SHIV)-specific immunity, which can control a pathogenic SHIV 89.6P challenge in macaques (Amara et al., 2001, Amara et al., 2002a, Amara et al., 2002b, Sadagopal et al., 2005). In this report we evaluate the potential of a clade B HIV-1 DNA/MVA/MVA (DMM) vaccine to elicit long-lived VV-specific immunity and confer protection from a lethal MPXV challenge. We longitudinally evaluate the magnitude of VV-specific cellular and humoral immunity raised by a DMM HIV-1 vaccine and compare these with the immune responses raised by a single Dryvax inoculation in rhesus macaques. At 3 years following vaccination, we conduct a MPXV challenge on a subset of the DMM- and Dryvax-vaccinated macaques to evaluate the potential of the HIV-1 DMM vaccine to confer protection against smallpox.
Results
Vaccinations
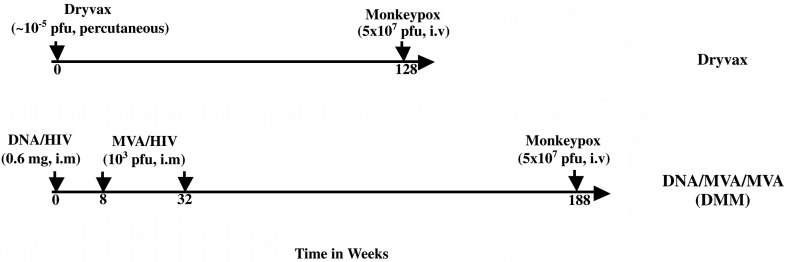
Two groups of macaques were used to evaluate the VV-specific immunity raised by MVA and Dryvax (Fig. 1 ). The first group, 15 macaques, received a DMM HIV-1 vaccine expressing HIV immunogens (Smith et al., 2004b). DNA (600 μg; JS2, JS7 or JS8) was inoculated on week 0 and MVA (1 × 108 pfu) on weeks 8 and 32. Four of the five macaques that received DNA/JS2 were challenged with 5 × 107 pfu of MPXV at 156 weeks post the last MVA inoculation. A second group of 10 macaques received a single inoculation of ∼ 1 × 105 pfu of Dryvax. Two of these 10 macaques were followed for 128 weeks and were challenged with MPXV.
Fig. 1.
Schematic for macaque trials. DNA/HIV and MVA/HIV are recombinant DNA and MVA vectors expressing HIV-1 vaccine inserts, respectively. i.v., intravenous; i.m, intramuscular; pfu, plaque forming units.
Temporal VV-specific CD4 and CD8 T cell responses elicited by the DMM and Dryvax vaccines
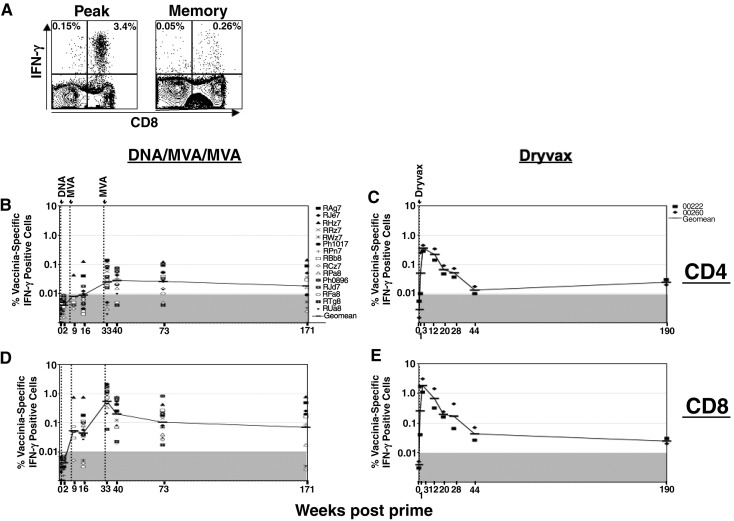
Intracellular cytokine staining was used to evaluate the temporal anti-vaccinia CD4 and CD8 T cell responses in macaques vaccinated with the DMM HIV-1 and Dryvax vaccines (Fig. 2 ). In the DMM-vaccinated macaques, as expected, VV-specific CD4 and CD8 cells were not detected until after the 1st MVA boost (Figs. 2B and D). At 1 week after the first MVA, low frequencies of responding CD8 T cells were detected in 4 of 5 tested macaques. By 8 weeks, detectable CD8 responses were present in 12 out of the 15 vaccinated macaques. CD4 responses were below the level of detection in the majority of the tested monkeys.
Fig. 2.
Temporal VV-specific cellular immunity elicited by the DMM and Dryvax vaccines. VV-specific IFN-γ producing CD4 and CD8 T cells were measured using an ICS assay. (A) A representative ICS assay. PBMC were stimulated with vaccinia as described in Materials and methods and stained for CD3, CD8 and IFN-γ. Cells were gated on lymphocytes based on the scatter pattern, followed by CD3 expression and analyzed for the expression of CD8 and IFN-γ. Cells in the right quadrants represent CD8 cells and left quadrants represent CD4 cells (CD3 positive, CD8 negative). The frequencies in the upper quadrants are IFN-γ producing cells as a % of total CD4 cells (left quadrants) or total CD8 cells (right quadrants). (B) VV-specific CD4 response in DMM-vaccinated macaques. (C) VV-specific CD4 response in Dryvax-vaccinated macaques. (D) VV-specific CD8 response in DMM-vaccinated macaques. (E) VV-specific CD8 response in Dryvax vaccinated macaques. Each symbol represents an individual macaque. There are 15 animals in the DMM group. For week 9, data are for 5 macaques and for week 174, data are for 10 macaques. The numbers on the graphs represent the geometric means for the groups at different time points. The shaded area on the graph represents the level of detection.
Peak cellular responses were observed after the 2nd MVA when both CD4 and CD8 responses expanded. At 1 week following the 2nd MVA boost, the magnitude of the CD8 response was 10–15-fold higher than the magnitude of the CD4 response. At this time, 11 out of 15 monkeys had a detectable CD4 response. These responses ranged from 0.02% to 0.14% of total CD4 cells and had a geometric mean frequency of 0.03%. In contrast to the CD4 response, CD8 responses were present in all of the macaques and ranged from 0.06% to 2.1% of total CD8 cells and had a geometric mean frequency of 0.53%.
The DMM vaccine-elicited VV-specific T cells were long-lived. CD4 responses underwent only minimal (0–4-fold) declines over the 3 years of study. The CD8 responses underwent an initial contraction of 2–25-fold after which they were stable. At 139 weeks post boost, 7 of 10 tested monkeys scored for responding CD4 cells with a geometric mean frequency of 0.02%, and 8 of 10 scored for responding CD8 cells with a geometric mean frequency of 0.07%. Two monkeys failed to score for responding CD4 or CD8 cells.
Analysis of vaccinia-specific T cell responses in macaques following a single Dryvax vaccination revealed higher levels of peak CD8 and CD4 T cell responses in Dryvax-vaccinated macaques than DMM-vaccinated macaques (Fig. 2, Fig. 4). However, long-term follow-up of vaccinia-specific T cell responses in 2 of the 10 Dryvax-vaccinated macaques revealed a greater contraction of CD8 (35–150-fold) and CD4 (250-fold) T cell responses by 44 weeks following vaccination in Dryvax-vaccinated macaques compared to the contraction of CD8 (2–25-fold) and CD4 (0–4-fold) T cell responses in DMM-vaccinated macaques resulting in similar levels of memory responses in both groups (Fig. 2).
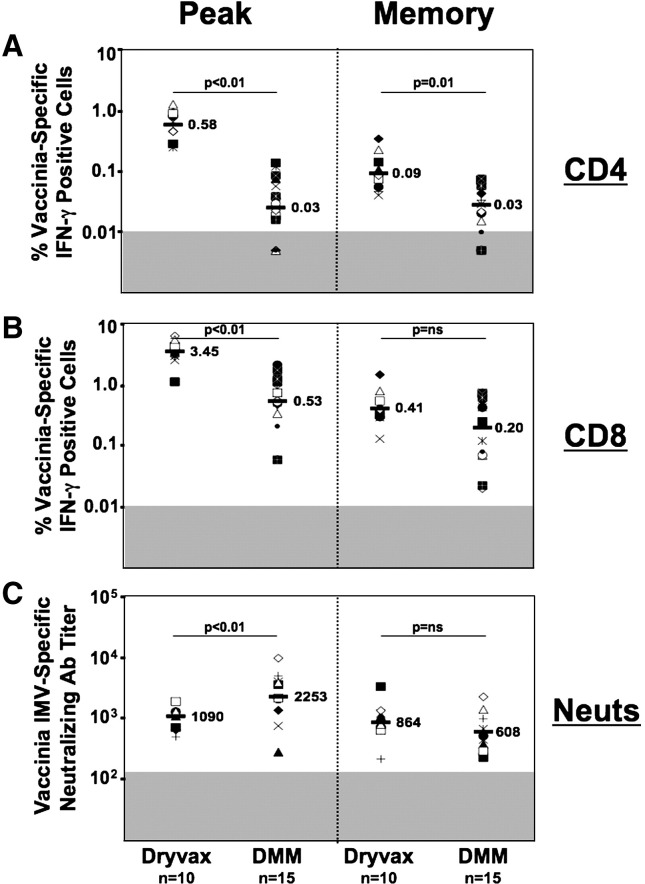
Fig. 4.
Comparison of VV-specific cellular and humoral responses elicited by Dryvax, and DMM vaccines in macaques. (A) VV-specific CD4 T cell response. (B) VV-specific CD8 T cell response. VV-specific IFN-γ producing CD4 and CD8 T cells were measured using an ICS assay. (C) Vaccinia virus MV-specific neutralizing antibody response. MV-specific neutralizing antibody response was measured using GFP based assay. Each symbol represents an individual macaque. The numbers on the graph represent the geometric means for the group at different time points. The shaded area on the graph represents the level of detection. ‘n’ represents the number of animals in the respective group. Peak CD4 and CD8 responses for Dryvax and DMM represent weeks 2 and 33 of the respective trials. Memory CD4 and CD8 responses for Dryvax and DMM represent weeks 12 and 40 of the respective trials. Peak neutralizing antibody response for Dryvax and DMM represents weeks 2 and 35 of the respective trials. Memory neutralizing antibody response for Dryvax and DMM represents weeks 84 and 167 of the respective trials. DMM, DNA/MVA/MVA.
Temporal VV-specific neutralizing antibody elicited by the DMM vaccine
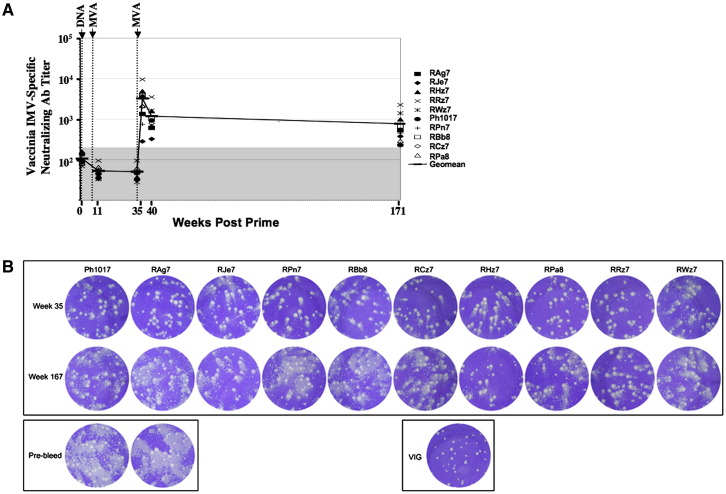
A GFP/flow cytometry based neutralization assay was used to evaluate neutralizing antibody to the mature virion (MV) form of vaccinia virus at various times following vaccination in macaques vaccinated with the DMM vaccine (Fig. 3A). Assays were performed using serum obtained at 3 weeks following the first MVA, and 3, 8 and 139 weeks following the second MVA. At 3 weeks following the first MVA, the titer of the neutralizing activity was below the level of detection. However, following the second MVA, these responses rapidly expanded. At 3 weeks following the second MVA, the neutralizing antibody titer ranged from 285 to 9615 and had a geomean titer of 2253. By 8 weeks, post the second MVA the titer had contracted about 2-fold. The DMM vaccine-elicited VV-specific neutralizing antibody response was long-lived and the titers remained fairly constant with minimal contraction for the next 131 weeks.
Fig. 3.
VV-specific neutralizing antibody elicited by HIV DMM vaccine. (A) Neutralizing antibody response against intracellular mature virion form of vaccinia virus measured using a GFP/flow based assay. Each symbol represents an individual macaque. The shaded area on the graph represents the background level of neutralizing activity observed in the sera of unvaccinated macaques. (B) Neutralizing antibody response against the extracellular form of vaccinia virus using a comet assay. VIG, vaccinia immunoglobulin.
Comet reduction assay was performed to evaluate the potential of VV-specific antibody to prevent the spread of extracellular form of vaccinia virus (EV) (Appleyard et al., 1971) (Fig. 3B). The comets represent satellite plaques that are caused by the spread of released virus by convection currents (Law et al., 2002). A strong comet reduction was observed using serum obtained at 3 weeks following the second MVA (week 35 in the trial). This activity persisted even at 135 weeks following the second MVA (week 167 in the trial) albeit at lower levels. Sera obtained from animals before immunization had no effect on the appearance of the diffuse comets.
Comparison of cellular and humoral immunity elicited by Dryvax and DMM vaccines
At the peak vaccine response Dryvax elicited about 10-times higher levels of CD4 response than the DMM vaccine (p < 0.01) (Fig. 4A). At the peak vaccine response, the frequency of CD4 T cells elicited by Dryvax ranged from 0.09% to 1.23% of total CD4 cells with a geometric mean of 0.58%. These frequencies are similar to what we previously reported for Dryvax-vaccinated humans (Amara et al., 2004). Unlike the CD4 response elicited by the DMM vaccine (Fig. 2), the CD4 response elicited by Dryvax underwent about a 5-fold contraction by 12 weeks as these cells differentiated into memory. After contraction, it still was 3-fold higher than the memory CD4 responses elicited by the DMM vaccine (p < 0.01). However, the longitudinal data obtained from the two Dryvax-vaccinated animals (Fig. 2) strongly suggest that the VV-specific CD4 response further contracts over time and will be maintained at levels that are similar to the levels observed in DMM-vaccinated macaques.
At peak vaccine response, Dryvax elicited a CD8 response that was about 5-fold higher than those elicited by the DMM vaccine (p < 0.01) (Fig. 4B). The CD8 response elicited by Dryvax ranged from 0.12% to 5.3% of total CD8 T cells and had a geometric mean frequency of 3.5%. Again, these frequencies are similar to what we previously reported for Dryvax-vaccinated humans (Amara et al., 2004). The peak CD8 response contracted in both groups as cells differentiated into memory.
In contrast to the lower peak cellular responses, the levels of vaccinia MV-specific neutralizing antibody elicited by the DMM vaccine were similar to the levels elicited by the Dryvax vaccine (p < 0.01) (Fig. 4C). At peak vaccine response, the titer of vaccinia MV-specific neutralizing antibody response raised by Dryvax had a geomean of 1090. These titers were about 2-fold lower than the titer of neutralizing antibody elicited by the DMM vaccine (p < 0.05). However, in the memory phase the Dryvax and DMM groups had similar levels of vaccinia MV-specific neutralizing antibody. At 85 weeks following Dryvax or 135 weeks following the second MVA, the titer of neutralizing antibody had a geomean of 864 and 608 in Dryvax and DMM groups, respectively.
Protection from a MPXV challenge by the Dryvax and DMM vaccines
Both the Dryvax and DMM vaccines conferred protection against a lethal MPXV challenge administered at 2 and one half (Dryvax) or 3 (DMM) years post vaccination (Table 1 ). Four of the 15 DMM-vaccinated macaques, 2 of the 10 Dryvax-vaccinated macaques and 2 unvaccinated naïve macaques were challenged with an intravenous inoculation of 5 × 107 pfu of MPXV. Only two macaques were included in the Dryvax and control group because of the availability of historical data for these two groups using the same challenge stock performed at the same facility (Edghill-Smith et al., 2005b). Viremia, measured as the copy number of MPXV genomes per ml of blood, and the number of skin lesions were used as measures of protection. In unvaccinated macaques, viral DNA was readily detected by day 2 and reached levels greater than 3 × 106 copies per ml of blood by day 8. These macaques developed numerous lesions, became severely ill and were euthanized by day 13. This outcome was similar to that of 5 historical control macaques that had been challenged with identical dose and route of challenge at the same facility (see Tables 2 and 3 of Edghill-Smith et al., 2005b). In contrast, the DMM-vaccinated macaques experienced transient low levels of viremia between days 5 and 13 and developed lesions. Three of the four DMM-vaccinated macaques developed greater than 200 lesions by day 8. However, these macaques rapidly controlled their viremia and skin lesions and were healthy by day 16. As expected (based on the data from the four historical Dryvax-vaccinated macaques; see Table 1 of Edghill-Smith et al., 2005b), the Dryvax-vaccinated macaques experienced low to undetectable levels of viremia. One of the Dryvax-vaccinated macaques developed greater than 200 lesions by day 8 but rapidly controlled by day 13.
Table 1.
Monkeypox viral DNA and number of skin pocks
| MPXV genomes/ml blood, log 10 on day |
Skin pocks on day |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 8 | 13 | 16 | 23 | 8 | 16 | |
| DNA/MVA | |||||||||
| RHz7 | < 3.7 | < 3.7 | < 3.7 | < 3.7 | 4.1 | < 3.7 | < 3.7 | > 200 | 0 |
| RAg7 | < 3.7 | < 3.7 | 4.3 | 5.1 | 3.9 | < 3.7 | < 3.7 | > 200 | 0 |
| RRz7 | < 3.7 | < 3.7 | < 3.7 | 3.9 | 4.2 | < 3.7 | < 3.7 | 0 | 0 |
| RJe7 | < 3.7 | < 3.7 | < 3.7 | 3.7 | < 3.7 | < 3.7 | < 3.7 | > 200 | 0 |
| Dryvax | |||||||||
| 00222 | < 3.7 | < 3.7 | < 3.7 | < 3.7 | < 3.7 | < 3.7 | < 3.7 | 0 | 0 |
| 00260 | < 3.7 | < 3.7 | 4.5 | < 3.7 | < 3.7 | < 3.7 | < 3.7 | > 200 | 0 |
| Control | |||||||||
| 00278 | < 3.7 | 4.5 | 4.6 | 8.2 | ** | ** | ** | > 200 | ** |
| Rhg8 | < 3.7 | 4.5 | 4.6 | 6.5 | ** | ** | ** | > 200 | ** |
** = Euthanized.
Rectal temperatures were taken on all of the animals at various times following challenge as shown in Table 2 . Macaques in the control group demonstrated the ‘typical’ temperature pattern that is normally seen for MPXV infections, where there is an elevation, peak and severe decrease prior to the time of euthanasia. In contrast, all vaccinated animals, except one animal in the DMM group (RHz7), did not demonstrate elevated temperatures. The macaque RHz7 had an elevated temperature at day 5 but returned to ‘normal’ temperature by day 8. Similarly, the vaccinated animals exhibited minimal weight loss (less than 4%), with the exception of RAg7 that exhibited a 6% weight loss by day 13. This animal was not euthanized because of the low viremia and small elevation in rectal temperature. However, this animal recovered rapidly by day 16.
Table 2.
Postchallenge temperature observations
| Rectal temperature (°F) on day |
|||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 8 | 13 | 16 | 23 | |
| DNA/MVA | |||||||
| RHz7 | 102.5 | 102.3 | 103.7 | 101.5 | 102.0 | 101.1 | 101.5 |
| RAg7 | 100.0 | 101.1 | 101.4 | 102.8 | 102.6 | 101.4 | 101.2 |
| RRz7 | 100.9 | 102.0 | 102.3 | 102.5 | 101.8 | 101.6 | 100.8 |
| RJe7 | 101.7 | 102.0 | 102.2 | 101.8 | 101.5 | 101.2 | 101.7 |
| Dryvax | |||||||
| 00222 | 100.6 | 100.5 | 101.4 | 101.6 | 100.8 | 100.5 | 101.0 |
| 00260 | 101.1 | 102.3 | 102.2 | 101.4 | 100.7 | 101.1 | 101.8 |
| Control | |||||||
| 00278 | 101.4 | 103.5 | 105.3 | 102.0** | |||
| RHg8 | 101.7 | 103.4 | 103.1 | 101.8** | |||
** = Euthanized.
Humoral and cellular immune response following MPXV challenge and correlates for protection
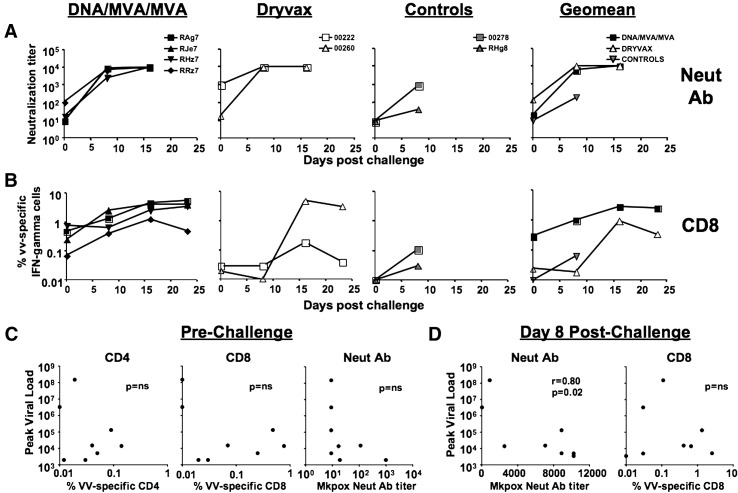
To address the relationship between postchallenge humoral and cellular immune responses and viral control, we measured the MPXV-specific neutralizing antibody and VV-specific CD8 and CD4 T cell responses at various times following challenge (Fig. 5 ). On the day of challenge, the levels of MPXV-specific neutralizing antibody were at or below the level of detection in all vaccinated and unvaccinated macaques except one DMM and one Dryvax macaque (Fig. 5A). Following MPXV challenge, the neutralizing antibody response rapidly expanded in all vaccinated but not unvaccinated macaques. At 8 days following challenge, the titer of MPXV neutralizing antibody was similar in both DMM and Dryvax groups ranging from 2500 to > 10,000. These titers were at least 10-fold higher compared to the titer in the control group (p < 0.01).
Fig. 5.
Cellular and humoral immunity, and correlates for protection following MPXV challenge. (A) MPXV-specific neutralizing antibody. Neutralization of monkeypox Zaire strain was performed using a plaque reduction assay. (B) VV-specific CD8 T cell response. VV-specific IFN-γ producing CD8 T cells were measured using an ICS assay. (C) Correlation between the peak viremia and immune responses on the day of challenge. (D) Correlation between the peak viremia and immune responses at day 8, postchallenge.
In contrast to the neutralizing activity, the levels of VV-specific CD8 T cell response were different between DMM and Dryvax groups following MPXV challenge (Fig. 5B). On the day of challenge, the VV-specific CD8 response was low to below the level of detection (0.01%) in vaccinated macaques. The magnitude of VV-specific CD8 response was higher in the DMM group compared to Dryvax group. At this time, 3 out of the 4 vaccinated macaques in the DMM group had CD8 response between 0.25 and 0.77% whereas these responses were at the level of detection in both macaques in the Dryvax group. Following MPXV challenge, the VV-specific CD8 T cells underwent a rapid expansion in the DMM group but not in the Dryvax group. At day 8 day following challenge, the VV-specific CD8 response in the DMM group ranged from 0.4 to 2.4% whereas these responses were below 0.03% in the Dryvax group. Similarly, the CD8 response was below 0.12% in the two unvaccinated macaques. However, by 16 days following challenge, the VV-specific CD8 T cell response expanded in the Dryvax group. In contrast to the CD8 response, the VV-specific CD4 response did not increase significantly either in the vaccinated or unvaccinated macaques following MPXV challenge (data not shown).
No correlation was observed between prechallenge VV-specific cellular immunity and postchallenge peak viremia (Fig. 5C). A weak inverse correlation (p = 0.07, r = − 0.7) between prechallenge MPXV-specific neutralizing antibody and peak viremia was observed. However, this correlation did not reach statistical significance could be due to the small group size. A strong inverse correlation between the levels of peak viral RNA and MPXV-specific neutralizing antibody at day 8 postchallenge was observed (p < 0.05, r = − 0.8) (Fig. 5D). This correlation was not observed between the levels of peak viral RNA and VV-specific CD8 T cell response at day 8 postchallenge (Fig. 5D).
Discussion
One of the hallmarks of vaccination using the replication competent vaccinia virus (Dryvax) is the elicitation of long-lived VV-specific cellular and humoral immunity (Amara et al., 2004, Crotty et al., 2003, Demkowicz et al., 1996, Hammarlund et al., 2003, Littaua et al., 1992). While the previous studies demonstrated that the replication defective MVA can elicit high titers of VV-specific neutralizing antibody (Earl et al., 2004), the longevity of this response has not been reported. Here, we evaluated the magnitude and longevity of VV-specific humoral and cellular immunity elicited by a recombinant MVA expressing HIV-1 proteins in macaques. The HIV-1 DMM vaccine elicited high frequency VV-specific CD8 and CD4 T cell responses and high titers of VV-specific neutralizing antibody. Two inoculations of MVA were required for eliciting these high levels of VV-specific immunity. The VV-specific cellular and humoral immunity elicited by the DMM vaccine was long-lived and could be detected even after 3 years following vaccination. Thus, our results clearly demonstrate that the replication defective MVA can elicit long-lived VV-specific cellular and humoral immunity in macaques.
Consistent with the long-lived VV-specific immunity elicited by the DMM vaccine, the DMM-vaccinated macaques were protected from a lethal MPXV challenge that was administered 3 years after the final immunization. Macaques vaccinated with the DMM vaccine developed transient low levels of viremia but rapidly controlled the infection. These results are similar to those observed with non-recombinant MVA in cynomolgus macaques (Earl et al., 2004, Stittelaar et al., 2005) suggesting that the observed low levels of viremia are not due to the use of a recombinant MVA, but an inherent property of MVA immunizations. Low levels of viremia were also observed in one of the two Dryvax-vaccinated macaques. Given the small group sizes it will be difficult to compare the level of protection between Dryvax- and DMM-vaccinated macaques. Nevertheless, our results clearly demonstrate that the HIV DMM vaccine can confer long-lived protection from a lethal MPXV challenge. Our study demonstrating the ability of MVA to elicit long-lived protection against a lethal monkeypox challenge is in good agreement with a parallel study testing for protection in rhesus macaques vaccinated with the MVA/MVA SHIV vaccine (Earl et al., 2007). In this study, the MPXV challenge was administered to macaques that had successfully controlled a SHIV-89.6P challenge.
For the first time, our study measured the VV-specific cellular immunity in addition to VV-specific humoral immunity following the MPXV challenge. This analysis revealed major differences in the magnitude of VV-specific CD8 T cell response between Dryvax- and DMM-vaccinated groups and provided insights about the correlates for protection. The postchallenge viral control was associated with early expansion of VV-specific neutralizing antibody rather than VV-specific CD8 or CD4 T cell response. At day 8 postchallenge all vaccinated macaques had high levels of MPXV-specific neutralizing antibody whereas, high frequency of VV-specific CD8 T cells were observed only in DMM-vaccinated macaques but not in Dryxax-vaccinated macaques. Despite the presence of low levels of VV-specific CD8 T cell responses, the Dryvax-vaccinated macaques were protected from MPXV challenge. These results strongly suggest a major role for neutralizing antibody and a more minor role for VV-specific CD8 and CD4 T cell response in protection against MPXV. These results are consistent with a previous study that demonstrated a major role for neutralizing antibody in protection against monkeypox by depleting specific T cell subsets prior to monkeypox challenge (Edghill-Smith et al., 2005b).
In contrast to the postchallenge response, following vaccination the DMM vaccine elicited lower levels of VV-specific cellular immune responses than Dryvax. At the peak of the vaccine-raised response, the DMM vaccine raised 5-fold lower frequencies of CD8 and 10-fold lower frequencies of CD4 cells than Dryvax. However, 2–3 years following vaccination, data obtained from very small number of macaques suggest similar or better persistence of VV-specific cellular immunity in DMM-vaccinated macaques compared to Dryvax-vaccinated macaques. Interestingly, these results in Dryvax-vaccinated macaques are consistent with our observation in humans following Dryvax vaccination (Amara et al., 2004). Previous studies comparing the immunogenicity of MVA and Dryvax in macaques either did not measure the VV-specific CD4 T cell response (Earl et al., 2004) or did not distinguish the responding cells into CD4 and CD8 T cells (Stittelaar et al., 2005). In contrast to the peak cellular immunity, the peak titers of VV-specific neutralizing and binding antibody raised by the DMM vaccine were similar, or higher, than the titers of VV-specific neutralizing antibody raised by Dryvax. This neutralizing activity was directed against both MV and EV forms of vaccinia virus. These results are consistent with the previous studies using Dryvax and wild type MVA in macaques (Earl et al., 2004, Stittelaar et al., 2005).
Following MPXV challenge, the number of pocks that we observed in recombinant MVA-vaccinated macaques was higher than observed in macaques vaccinated with non-recombinant MVA by Earl et al. (Earl et al., 2004, Stittelaar et al., 2005). In our study, 3 out of the four MVA-vaccinated macaques experienced greater than 200 pocks, whereas the number of pocks was below 40 in the study by Earl et al. Similarly, one of the two Dryvax-vaccinated macaques also had greater than 200 pocks that was higher than that was previously reported for Dryvax-vaccinated macaques (Edghill-Smith et al., 2005b). Interestingly, the two vaccinated macaques (one from each group) that did not exhibit skin pocks also had higher levels of MPXV-specific neutralizing antibody at the time of challenge. These results suggest an inverse relationship between the prechallenge neutralizing antibody and postchallenge pocks. In addition, there was no correlation between monkeypox viremia and number of skin pocks in our study. There are multiple differences between our study and prior studies using MVA or Dryvax. We used recombinant MVA in rhesus macaques (Macaca mullata) and challenged 3 years after the final immunization. The prior MVA study was conducted in cynomolgus macaques (Macaca fascicularis) using wild type MVA and challenges were performed at 8 weeks after the final immunization at a different facility using a different challenge stock. The prior Dryvax study was conducted in rhesus macaques at 8 weeks after the final immunization at the same facility using the same challenge stock. We suggest that the long time after challenge would have contributed to the observed higher levels of skin pocks in our study due to the reduction in neutralizing antibody titers that is known to occur over time. This is also supported by the fact that VV-specific neutralizing antibody seems to play a major role in protection against MPXV challenge.
In conclusion, our results clearly demonstrate that the HIV-1 DMM vaccine elicits long-lived VV-specific cellular and humoral immune responses that confer durable protection from a lethal MPXV challenge. They also demonstrate that VV-specific humoral immunity, but not cellular immunity, plays a major role for viral control post MPXV challenge. If the HIV-1 DNA/MVA vaccine proves successful in controlling HIV/AIDS in humans, our results suggest that it may also be able to serve as a dual vaccine for protection against both HIV and smallpox.
Materials and methods
DNA/HIV vaccines
The JS2, JS7 and JS8 DNA constructs used for DNA priming were made in our pGA expression vectors that use the CMV immediate early promoter and the bovine growth hormone polyadenylation sequence to express RNAs (Amara et al., 2005, Smith et al., 2004a, Smith et al., 2004b). JS2 and JS7 express Gag, PR, RT, Env, Tat, Rev and Vpu sequences of clade B HIV-1 by subgenomic splicing of the RNA expressed by the vaccine insert, and JS8 expresses a codon-optimized Gag. All three plasmids were grown in DH5α, purified using Qiagen endonuclease-free Giga preps, and dissolved in Mg++ and Ca++ free phosphate buffered saline at 0.3 mg per ml.
MVA/HIV vaccine
MVA/HIV 48 was used for boosting (Wyatt et al., 2004b). This MVA expresses Gag, PR, RT and a C-terminal truncated form of Env that are from the same sequences used to construct JS7. MVA/HIV 48 was purified by sedimentation through a sucrose pad and was resuspended in phosphate buffered saline at 1 × 108 pfu per ml.
Macaques and vaccine trials
DMM HIV vaccine trial
Nine Chinese and 6 Indian rhesus macaques were primed with DNA on week 0 and boosted with MVA/HIV 48 on weeks 8 and 32 as previously reported (Smith et al., 2004b). Five macaques received JS2 DNA, 5 received JS7 DNA and 5 received JS8 DNA. Each macaque received 0.6 mg of a test DNA i.m. using a needle and syringe, one half delivered to the upper lateral right thigh and one half delivered to the upper lateral left thigh. All macaques were boosted in the upper lateral right thigh i.m. with 1 ml of 1 × 108 pfu of MVA/HIV 48.
Dryvax trial
Ten Indian rhesus macaques were primed with Dryvax (the current smallpox vaccine) on week 0 using the standard human dose. The Dryvax vaccine was given by scarification, as recommended by the Centers for Disease Control and Prevention. Briefly, macaques were inoculated with a bifurcated needle that held a drop of vaccine virus at a concentration of approximately 1 × 108 pfu/ml and that was pressed 15 times into the skin of the abdomen. This dose represents about 1 × 105 pfu as has been estimated previously (Frey et al., 2002).
General procedures for animal care and housing were in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals (1996) and the Animal Welfare Standards incorporated in 9CFR Part 3, 1991.
Intracellular Cytokine Staining (ICS) assay
ICS assays were performed as described previously (Amara et al., 2001, Amara et al., 2004, Speller and Warren, 2002). Briefly, approximately 1 × 106 PBMC were stimulated in 5 ml polypropylene tubes in RPMI containing 10% fetal bovine serum, anti-human CD28, anti-human CD49d (1 μg per ml each, Pharmingen, Inc. San Diego, CA) in 100 μl. VV-specific T cells were measured using vaccinia virus strain WR. Approximately 2 × 106 pfu of vaccinia virus strain WR (multiplicity of infection of 2, MOI) was added in a volume of 100 μl. After 12 h of incubation at 37 °C, 900 μl of RPMI containing 10% FBS and monensin (10 μg/ml) was added and cells were cultured for an additional 3 h at 37 °C at an angle of 5°. At the end of stimulation, cells were surface stained with fluorchrome conjugated antibodies to CD8 (clone SK1, Becton Dickinson) at 8°–10 °C for 30 min, washed once with cold PBS containing 2% FBS and fixed and permeabilized with Cytofix/Cytoperm solution (Pharmingen, Inc.). Cells were then incubated with fluorchrome conjugated antibodies to macaque CD3 (clone FN-18, Biosource Int.) and IFN-γ (clone B27, Pharmingen) in Perm wash solution (Pharmingen) for 30 min at 4 °C. Cells were washed twice with Perm wash, once with plain PBS and resuspended in 1% formalin in PBS. Approximately 200,000 lymphocytes were acquired on the FACScaliber and analyzed using FloJo software (Treestar Inc. San Carlos, CA). Lymphocytes were identified based on their scatter pattern and CD3+, CD8− cells were considered as CD4 positive T cells and CD3+, CD8+ cells were considered as CD8 positive T cells. Using this assay, we could detect VV-specific CD4 and CD8 T cells as low as 0.01% of the respective total cells. In unvaccinated controls and pre-vaccination bleeds of vaccinated macaques, the frequencies of VV-specific CD4 and CD8 T cells were below 0.01%.
Humoral responses
Neutralization assay against the intracellular mature form (MV) form of vaccinia virus was performed using a recombinant vaccinia virus strain WR that expresses GFP as previously described (Earl et al., 2003). Comet reduction assay was performed to measure the neutralizing antibodies against extracellular form (EV) of vaccinia virus. Briefly, BSC-1 cells were infected with the vaccinia virus strain IHD-J, which produces comet-like satellite plaques due to the release of large amounts of extracellular virus. After 2 h, monolayers were washed three times and overlaid with medium containing serum samples diluted 1:66. After 36–40 h, cells were stained with crystal violet and photographed.
Neutralizing antibody titer against the MPXV Zaire strain was performed using a plaque reduction assay. Briefly, monkeypox virus strain Zaire 79 was diluted in complete medium (DMEM containing 2% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin and 10 mM HEPES) to give 2000 PFU/ml. Aliquots of this viral suspension (100 μl) were incubated with an equal volume of heat inactivated serum diluted in complete medium (serum samples were heat activated at 56 °C for 30 min, before dilution) for 15 h at 4 °C and then adsorbed to confluent Vero E6 cell monolayers in 24-well plates for 1 h in a 37 °C 5% CO2 incubator. A 0.5-ml semisolid overlay (MEM, 0.5% methyl cellulose, 2% heat-inactivated FBS, antibiotics) was added to each well. Plates were incubated in a 37 °C 5% CO2 incubator for 3 days. Cell monolayers were stained with 0.25 ml of crystal violet staining solution (0.1% crystal violet, 70% ethanol) for 1 h at room temperature. Plaques were counted and the percent neutralization was calculated relative to the number of plaques in the absence of Ab. Titers represent the reciprocal of the highest dilution resulting in a 50% reduction in the number of plaques.
Monkeypox virus (MPXV) challenge
MPXV challenges were performed at Southern Research Institute (Frederick, Maryland, USA). Macaques were challenge intravenously with 5 × 107 pfu of MPXV virus (Zaire strain). The criteria used to assess whether euthanasia should be implemented included development of pock lesions, MPXV viremia, pyrexia, anorexia, weight loss, lethargy and moribundity. These were used in conjunction with other parameters, such as physical observations and veterinarian recommendation. Generally, macaques were euthanized if the temperature was consistently above 103 °F, weight loss greater than 4%, and viral loads greater than 106 DNA genome copies per ml blood. In some instances, the presence of too numerous to count lesion numbers within the oral cavity was taken into consideration since this hinders eating and leads to weight loss.
Statistical analysis
The Wilcoxon rank sum test was used to compare differences in T cell and antibody responses between groups. Spearman rank-correlation was used to determine the correlation between viral load and neutralizing antibody or CD8 response.
Acknowledgments
We thank J. Herndon for help with statistical analysis and H. Drake-Perrow for outstanding administrative support. We are thankful to The Yerkes Division of Research Resources for the consistent excellence of veterinary care and pathology support. This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases grants R21 AI53488, R01 AI57029 to RA and P01 AI49364 to HR and Yerkes National Primate Research Center base grant, P51 RR00165. Support to PE, LW and BM was provided by NIAID, Division of Intramural Research.
References
- Amara R.R., Villinger F., Altman J.D., Lydy S.L., O'Neil S.P., Staprans S., Montefiori D.C., Xu Y., Herndon J.G., Wyatt L.S., Candido M.A., Kozyr N.L., Earl P.L., Smith J.M., Ma H.-L., Grimm B.D., Hulsey M.L., Miller J., McClure H.M., McNicholl J.M., Moss B., Robinson H.L. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- Amara R.R., Smith J.M., Staprans S., Montefiori D., Villinger F., Altman J.D., O'Neil S.P., Kozyr N.L., Xu Y., Wyatt L., Earl P.L., Herndon J.G., McNicholl J.M., McClure H.M., Moss B., Robinson H.L. Critical role for Env as well as Gag-Pol for the control of a pathogenic SHIV challenge by a DNA/rMVA Vaccine. J. Virol. 2002;76(12):6138–6146. doi: 10.1128/JVI.76.12.6138-6146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara R.R., Villinger F., Staprans S., Altman J.D., Montefiori D., Kozyr N.L., Xu Y., Wyatt L., Earl P.L., Herndon J.G., McClure H.M., Moss B., Robinson H.L. Different patterns of immune responses but similar control of a mucosal immunodeficiency virus challenge by MVA and DNA/MVA vaccines. J. Virol. 2002;76:7625–7631. doi: 10.1128/JVI.76.15.7625-7631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara R.R., Nigam P., Sharma S., Liu J., Bostik V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of cD4 than CD8 T cells. J. Virol. 2004;78(8):3811–3816. doi: 10.1128/JVI.78.8.3811-3816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara R.R., Sharma S., Patel M., Smith J., Chennareddi L., Herndon J.G., Robinson H. Studies on the cross-clade and cross-species conservation of HIV-1 Gag-Specific CD8 and CD4 T cell responses elicited by a clade B DNA/MVA vaccine in macaques. Virology. 2005;334(1):124–133. doi: 10.1016/j.virol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Antoine G., Scheiflinger F., Dorner F., Falkner F.G. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244(2):365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- Appleyard G., Hapel A.J., Boulter E.A. An antigenic difference between intracellular and extracellular rabbitpox virus. J. Gen. Virol. 1971;13(1):9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- Barouch D.H., Santra S., Kuroda M.J., Schmitz J.E., Plishka R., Buckler-White A., Gaitan A.E., Zin R., Nam J.H., Wyatt L.S., Lifton M.A., Nickerson C.E., Moss B., Montefiori D.C., Hirsch V.M., Letvin N.L. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 2001;75(11):5151–5158. doi: 10.1128/JVI.75.11.5151-5158.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov I.M., Earl P., Dzutsev A., Kuznetsov V.A., Lemon M., Wyatt L.S., Snyder J.T., Ahlers J.D., Franchini G., Moss B., Berzofsky J.A. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. U.S.A. 2003;100(16):9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard T.J., Alcami A., Andrea P., Smith G.L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 1998;79(Pt. 5):1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- Crotty S., Felgner P., Davies H., Glidewell J., Villarreal L., Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Demkowicz W.E., Jr., Littaua R.A., Wang J., Ennis F.A. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J. Virol. 1996;70(4):2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P.L., Wyatt L.S., Montefiori D.C., Bilska M., Woodward R., Markham P.D., Malley J.D., Vogel T.U., Allen T.M., Watkins D.I. Comparison of vaccine strategies using recombinant env–gag–pol MVA with or without an oligomeric env protein boost in the SHIV rhesus macaque model. Virology. 2002;294(2):270–281. doi: 10.1006/viro.2001.1345. [DOI] [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Moss B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J. Virol. 2003;77(19):10684–10688. doi: 10.1128/JVI.77.19.10684-10688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., Eisenberg R.J., Hartmann C.J., Jackson D.L., Kulesh D.A., Martinez M.J., Miller D.M., Mucker E.M., Shamblin J.D., Zwiers S.H., Huggins J.W., Jahrling P.B., Moss B. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Montefiori D.C., Byrum R., Piatak M., Lifson J.D., Amara R.A., Robinson H.L., Huggins J.W., Moss B. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology. 2007;366:84–97. doi: 10.1016/j.virol.2007.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edghill-Smith Y., Bray M., Whitehouse C.A., Miller D., Mucker E., Manischewitz J., King L.R., Robert-Guroff M., Hryniewicz A., Venzon D., Meseda C., Weir J., Nalca A., Livingston V., Wells J., Lewis M.G., Huggins J., Zwiers S.H., Golding H., Franchini G. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect Dis. 2005;191(3):372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E., Reimann K.A., Franchini G. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- Enserink M. Bioterrorism. In search of a kinder, gentler vaccine. Science. 2002;296(5573):1594. doi: 10.1126/science.296.5573.1594. [DOI] [PubMed] [Google Scholar]
- Frey S.E., Couch R.B., Tacket C.O., Treanor J.J., Wolff M., Newman F.K., Atmar R.L., Edelman R., Nolan C.M., Belshe R.B., and the National Institute of Allergy and Infectious Diseases Smallpox Vaccine Study Group Clinical responses to undiluted and diluted smallpox vaccine. N. Engl. J. Med. 2002;346(17):1265–1274. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- Gani R., Leach S. Transmission potential of smallpox in contemporary populations. Nature. 2001;414(6865):748–751. doi: 10.1038/414748a. [DOI] [PubMed] [Google Scholar]
- Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;10.1038/nm917:1–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Henderson D.A. The looming threat of bioterrorism. Science. 1999;283(5406):1279–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- Henderson D.A. In: Smallpox and Vaccinia. 3rd ed. Plotkin S.A., Orenstein W.A., editors. W.B. Saunders Co; Philadelphia, PA: 1999. (Vaccines). [Google Scholar]
- Hirsch V.M., Fuerst T.R., Sutter G., Carroll M.W., Yang L.C., Goldstein S., Piatak M., Jr., Elkins W.R., Alvord W.G., Montefiori D.C., Moss B., Lifson J.D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 1996;70(6):3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M., Hollinshead R., Smith G.L. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 2002;83(Pt. 1):209–222. doi: 10.1099/0022-1317-83-1-209. [DOI] [PubMed] [Google Scholar]
- Littaua R.A., Takeda A., Cruz J., Ennis F.A. Vaccinia virus-specific human CD4+ cytotoxic T-lymphocyte clones. J. Virol. 1992;66(4):2274–2280. doi: 10.1128/jvi.66.4.2274-2280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack T.M., Noble J., Jr., Thomas D.B. A prospective study of serum antibody and protection against smallpox. Am. J. Trop. Med. Hyg. 1972;21(2):214–218. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- Mayr A., Stickl H., Muller H.K., Danner K., Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's transl) Zentralbl. Bakteriol. [B] 1978;167(5-6):375–390. [PubMed] [Google Scholar]
- O'Connell C.J., Karzon D.T., Barron A.L., Plaut M.E., Ali V.M. Progressive vaccinia with normal antibodies: a case possibly due to deficient cellular immunity. Ann. Intern. Med. 1964;60:282–289. doi: 10.7326/0003-4819-60-2-282. [DOI] [PubMed] [Google Scholar]
- Panchanathan V., Chaudhri G., Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J. Virol. 2006;80(13):6333–6338. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J.C., Gherardi M.M., Esteban M. Biology of attenuated modified vaccinia virus ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the western reserve strain and advantages as a vaccine. J. Virol. 2000;74(2):923–933. doi: 10.1128/jvi.74.2.923-933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield R.R., Wright D.C., James W.D., Jones T.S., Brown C., Burke D.S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N. Engl. J. Med. 1987;316(11):673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- Sadagopal S., Amara R.R., Montefiori D.C., Wyatt L.S., Staprans S.I., Kozyr N.L., McClure H.M., Moss B., Robinson H.L. Signature for long-term vaccine-mediated control of a simian and human immunodeficiency virus 89.6P challenge: stable low-breadth and low-frequency T-cell response capable of coproducing gamma interferon and interleukin-2. J. Virol. 2005;79(6):3243–3253. doi: 10.1128/JVI.79.6.3243-3253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J.K., Mitra A.C., Mukherjee M.K. The minimum protective level of antibodies in smallpox. Bull. World Health Organ. 1975;52(3):307–311. [PMC free article] [PubMed] [Google Scholar]
- Smith J.M., Amara R.R., McClure H.M., Patel M., Sharma S., Yi H., Chennareddi L., Herndon J.G., Butera S.T., Heneine W., Ellenberger D.L., Parekh B., Earl P.L., Wyatt L.S., Moss B., Robinson H.L. Multiprotein HIV type 1 clade B DNA/MVA vaccine: construction, safety, and immunogenicity in Macaques. AIDS Res. Hum. Retroviruses. 2004;20(6):654–665. doi: 10.1089/0889222041217419. [DOI] [PubMed] [Google Scholar]
- Smith J.M., Rao Amara R., Campbell D., Xu Y., Patel M., Sharma S., Butera S.T., Ellenberger D.L., Yi H., Chennareddi L., Herndon J.G., Wyatt L.S., Montefiori D., Moss B., McClure H.M., Robinson H.L. DNA/MVA vaccine for HIV Type 1: effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS Research and Human Retroviruses. 2004;20(12):1335–1347. doi: 10.1089/aid.2004.20.1335. [DOI] [PubMed] [Google Scholar]
- Speller S.A., Warren A.P. Ex vivo detection and enumeration of human antigen-specific CD8+ T lymphocytes using antigen delivery by a recombinant vaccinia expression vector and intracellular cytokine staining. J. Immunol. Methods. 2002;262(1-2):167–180. doi: 10.1016/s0022-1759(02)00025-x. [DOI] [PubMed] [Google Scholar]
- Stickl H., Hochstein-Mintzel V., Mayr A., Huber H., Schafer H., Holzner A. MVA vaccination against smallpox: clinical trials of an attenuated live vaccinia virus strain (MVA) Dtsch. Med. Wochenschr. 1974;99:2386–2392. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H.M., Niesters H.G.M., van Doornum G., van der Zeijst B.A.M., Mateo L., Chaplin P.J., Osterhaus A.D.M.E. Modified vaccinia virus ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 2005;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter G., Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. U.S.A. 1992;89(22):10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter G., Wyatt L.S., Foley P.L., Bennink J.R., Moss B. A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994;12(11):1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Weltzin R., Liu J., Pugachev K.V., Myers G.A., Coughlin B., Blum P.S., Nichols R., Johnson C., Cruz J., Kennedy J.S., Ennis F.A., Monath T.P. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat. Med. 2003;9(9):1125–1130. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- Wyatt L.S., Shors S.T., Murphy B.R., Moss B. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine. 1996;14(15):1451–1458. doi: 10.1016/s0264-410x(96)00072-2. [DOI] [PubMed] [Google Scholar]
- Wyatt L.S., Earl P.L., Eller L.A., Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. U.S.A. 2004;101(13):4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt L.S., Earl P.L., Liu J.Y., Smith J.M., Montefiori D.C., Robinson H.L., Moss B. Multiprotein HIV type 1 clade B DNA and MVA vaccines: construction, expression, and immunogenicity in rodents of the MVA component. AIDS Res. Hum. Retroviruses. 2004;20(6):645–653. doi: 10.1089/0889222041217428. [DOI] [PubMed] [Google Scholar]