Abstract
The commonly used inhaled anesthetic isoflurane has been shown to be both neuroprotective and neurotoxic in various cell cultures and animal models. We hypothesize that, like cerebral ischemia, isoflurane is inherently neurotoxic. Limited exposure of isoflurane provides neuroprotection via induction of endogenous neuroprotective mechanisms (preconditioning), while prolonged exposure of isoflurane induces neurotoxicity directly by its inherent neurotoxic effects. To test this hypothesis, we treated rat primary cortical neurons at different days in vitro (DIV) and rat pheochromocytoma neurosecretory (PC12) cells with or without Alzheimer's mutated presenilin-1 (PS1) with 2.4% isoflurane for 24 hr to induce cell damage determined by both MTT (3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) reduction and LDH (lactate dehydrogenase) release assays. For isoflurane preconditioning, we treated the above cells with isoflurane at 0.6%, 1.2% and 2.4% for 60 minutes, 4 hrs prior to a prolonged exposure of 2.4% isoflurane for 24 hr. One hr of preconditioning with isoflurane dose-dependently inhibited neurotoxicity induced by 2.4% isoflurane for 24 hr in both primary cortical neurons and PC12 cells. This neuroprotection was most dramatically observed in matured cortical neurons (DIV 16) and PC12 cells with over expression of Alzheimer's mutated PS1 (L286V). Preconditioning L286V PC12 cells with equivalent two minimal alveolar concentrations (MAC) of halothane (1.5%), but not sevoflurane (4%), also abolished the neurotoxicity induced by 2.4% isoflurane for 24 hr. Overall, these results suggest that isoflurane may be both neuroprotective and neurotoxic, depending on the exposure concentrations and duration.
Keywords: Anesthesia, Cell Death, Alzheimer's disease, Preconditioning, Neuroprotection
Inhaled anesthetics, especially isoflurane, are commonly used to maintain general anesthesia for various surgeries or procedures in patients, as well as in animal models for studies related to neuroprotection or neurotoxicity. Isoflurane has long been considered a neuroprotective agent in various cell cultures and animal models [13-15;24;25]. Increasing evidence suggests isoflurane also induces neuronal apoptosis dose- and time-dependently in various cell cultures and in the developing brains in different animal models [5;8;12;16;19-21]. Therefore for both clinical anesthesiologists and basic research scientists, it is important to clarify whether isoflurane and other inhaled anesthetics should be considered neuroprotective or neurotoxic. In this study, we tested a hypothesis that isoflurane is inherently neurotoxic. Like ischemia preconditioning, short exposure of isoflurane protects against neurotoxicity induced by prolonged exposure of isoflurane in both rat primary cortical neurons at different maturation ages and pheochromocytoma neurosecretory (PC12) cells with or without over expression of Alzheimer's mutated presenilin-1 (PS1).
The use of pregnant rats for primary cortical neuronal culture was approved by the Institutional Animal Care and Use (IACUC) at the University of Pennsylvania. Primary cultures of cortical neurons were prepared from the dissociated cortices of rat fetus at embryonic day 18 using a protocol previously described [16]. Cortices were dissected from embryonic brain, and meninges were removed from the tissues. The cells were dissociated by trypsinization and trituration, followed by DNase treatment. The dissociated cells were resuspended in serum-free B27/neurobasal medium and were plated at a density of 1×105 cells/cm2 on poly-D-lysine-coated 96-well plates. Cultures were maintained in serum-free B27/neurobasal medium in a humidified atmosphere (5% CO2, 95% air) at 37°C. More than 95% of the cells present on fifth day in vitro (DIV 5) differentiate into neurons, as characterized by the appearance of long neurites expressing neurofilament protein. Half of the medium was changed every fourth day. Cerebral cortical neurons at different maturation (DIV 3, 7 and 16) were used for the experiments. Rat pheochromocytoma cells (PC12) transfected with wild type PS1 (WT), vector alone control (Vector) and point mutated PS1 (L286V) were cultured as previously described [4;7;16]. As a short summary, the cells were maintained in DMEM medium (Invitrogen Corporation, Grand Island, NY, USA) supplemented with 10% heat-inactivated horse serum (Invitrogen Life Technologies, Carlsbad, CA, USA), 5% fetal calf serum (Hyclone Laboratories, Logan, UT, USA), 200 μg/ml G418 (Mediatech, Inc., Herrden, VA, USA) and penicillin/streptomycin (Invtrogen Life Technologies, Carlsbad, CA, USA). Monolayer cultures at a density of 0.3×105 cells/cm2 were incubated in plastic flasks precoated with 0.01% poly-l-ornithine (Sigma-Aldrich, St. Louis, MO, USA) in a 95% air, 5% CO2 humidified atmosphere at 37°C. The culture medium was changed every 48 hr. The transfection of the WT and mutant PS1 has been described and confirmed in detail previously [6;7].
Rat primary cortical neurons and different types of PC12 cells grown on 24-well plates were exposed to inhaled anesthetics in a gas-tight chamber inside the culture incubator (Bellco Glass, Inc., Vineland, NJ, USA), with humidified 5%CO2/21%O2/balanced N2 (AirGas East, Bellmawr, NJ, USA) going through a calibrated agent-specific vaporizer as described previously [16]. Gas phase concentrations in the gas chamber were checked with infrared absorbance of the effluent gas, and constantly monitored and maintained at the designed concentration throughout experiments, using an infrared Ohmeda 5330 agent monitor (Coast to Coast Medical, Fall River, MA, USA). The neurotoxic model of isoflurane was established by exposing cells in the chamber to 2.4% isoflurane for 24 hr. The 1 hr preconditioning of neurons with isoflurane at different concentrations or equivalent 2 minimal alveolar concentrations (MAC) of isoflurane (2.4%), halothane (1.5%) or sevoflurane (4%) was carried out 4 hrs prior to the initiation of prolonged exposure of 2.4% isoflurane for 24 hr.
Neurotoxicity was assessed by the MTT (3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) reduction and LDH (lactate dehydrogenase) release assays exactly as we described previously [16]. The MTT tetrazolium compound was bioreduced by cells into a colored formazan product, and decrease of MTT reduction of cells represented an early stage of cell damage. LDH release assay determined the cell plasma membrane integrity by measuring the degree of the LDH enzyme released from the cells into the culture medium, representing a relatively late stage of cell damage. Both assays have been frequently used to determine cytotoxicity in different cell cultures models [16-18]. The results of MTS reduction and LDH release assays were expressed as percentage of control without anesthetic treatment.
All data were expressed as mean ± SEM and were analyzed by one-way ANOVA followed by Newman-Keuls multiple comparison tests. P values less than 0.05 were considered statistically significant.
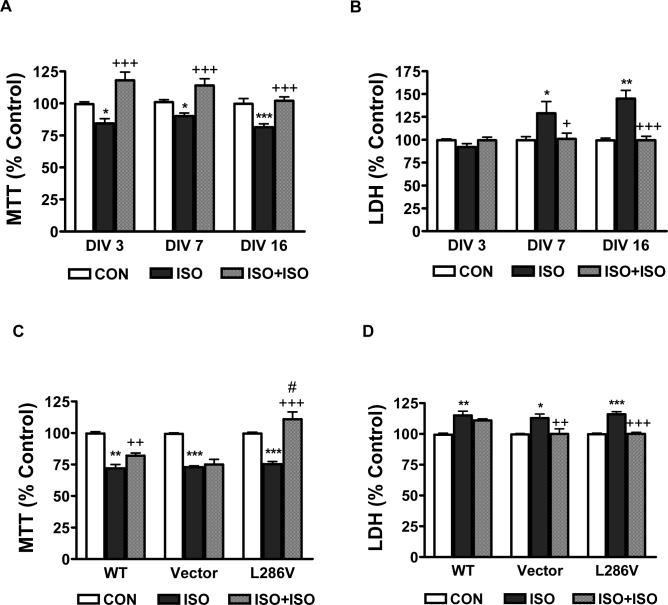
Consistent with our previous publication [16], 2.4% isoflurane for 24 hr induced significant MTT reduction and LDH release in both primary cortical neurons (Figure 1 A and B) and different type of PC12 cells (Figure 1 C and D). In addition, mature primary cortical neurons seemed to be more vulnerable to isoflurane-induced late cell damage (LDH release) than the immature neurons (Figure 1 B, DIV 3 vs. DIV 16), although this difference did not exist for the early cell damage (MTT reduction, Figure 1 A). Preconditioning primary cortical neurons and different types of PC12 cells with 2.4% isoflurane for 1 hr abolished the neurotoxicity induced by the subsequent prolonged exposure of 2.4% isoflurane for 24 hr, as determined by both MTT reduction (Figure 1 A and C, ISO vs. ISO+ISO) and LDH release (Figure 1 B and D, ISO vs. ISO+ISO) assays. Isoflurane had greater preconditioning potency in L286V than in WT or Vector control PC12 cells (Figure 1C). In addition, short exposure of isoflurane preconditioning not only abolished the neurotoxicity induced by prolonged exposure of isoflurane but also enhanced cell survival above the control level (Figure 1 A and C).
Figure 1. Preconditioning with short exposure of isoflurane inhibited neurotoxicity induced by prolonged exposure of isoflurane.
Both rat primary cortical neurons at different day in vitro (DIV, A and B) and PC12 cells transfected with wild type (WT), vector or mutated (L286V) Alzheimer's presenilin -1 (C and D) were treated with 2.4% isoflurane for 24 hr with (ISO+ISO) or without (ISO) 1 hr preconditioning at 4 hr before the prolonged exposure. Data represents mean ± SEM from minimum 12 repeats of at least three separate experiments. *, ** or *** means P<0.5, P<0.01 or P<0.001 compared to control (CON). ++ or +++ means P<0.5 or P<0.001 compared to ISO. # means P<0.05 compared to CON (C).
We further tested if isoflurane preconditioned cells dose-dependently, similar to the way it induced neurotoxicity [16]. Isoflurane's preconditioning ability significantly decreased as its concentration dropped (Figure 2A), and 0.6% isoflurane demonstrated no preconditioning protection against L286V PC12 cell damage induced by 2.4% isoflurane for 24 hr. To examine the possible cross preconditioning among different inhaled anesthetics, we determined the preconditioning potential of equi-potent 2 MAC of halothane (1.5%) or sevoflurane (4%) and compared to that of isoflurane. Preconditioning L286V PC12 cells with 1.5% halothane but not 4% sevoflurane significantly inhibited MTT reduction induced by 2.4% isoflurane for 24 hr (Figure 2B), suggesting the inhaled anesthetics may have cross preconditioning and the potency for preconditioning among inhaled anesthetics may be different.
Figure 2. Comparison of preconditioning potency.

A. Short exposure of isoflurane (ISO) preconditioned L286V PC12 cells dose-dependently. PC12 cells transfected with mutated Alzheimer' presenilin-1 (L286V) were preconditioned with 0.6%, 1.2% and 2.4% isoflurane for 1 hr, then treated with 2.4% isoflurane for 24 hr. B. Equivalent 2 Minimal Alveolar Concentration (MAC) of isoflurane (2.4% ISO), halothane (1.5% HAL) and sevoflurane (4% SEVO) were used to precondition (PRE) L286 PC12 cells for protection against MTT reduction induced by 2.4% isoflurane for 24 hr. Data represents mean ± SEM from minimum 12 repeats for at least three experiments. *** means P<0.001 compared to control (CON). + or +++ means P<0.5 or P<0.001 compared to ISO treatment alone without ISO preconditioning (ISO). # means P<0.05 compared to control.
Isoflurane preconditions cells and induces endogenous cytoprotective mechanisms, including activation of the adenosine A1 receptor, protein kinase C, ATP-dependent potassium channels, elevation of mitochondrial reactive oxygen species (ROS) [9] or modulation of apoptotic regulatory proteins (e.g. elevation of Bcl-2/Bax ratio) [25]. In addition, mild calcium release from the ER and moderate elevation of cytosolic calcium concentration by isoflurane at low concentration and short duration may trigger the ER stress response, marked by the expression of genes characterizing the well-known “preconditioning” effect [1;2]. Prolonged exposure of isoflurane at high concentration, producing extensive and prolonged calcium release from ER may deplete ER calcium and shut down protein synthesis leading to “cytotoxicity” effects [3;16;23].
Our results are most consistent with the hypothesis that isoflurane may be both neuroprotective and neurotoxic, depending on the concentrations and exposure durations. Similar to its dose-dependent neurotoxic effects [8;16], the preconditioning effects of isoflurane were also dose-dependent. , In addition, the preconditioning potency of inhaled anesthetics may be related to their potency of induced neurotoxicity. Sevoflurane has been shown to be much less potent than isoflurane for induction of cytotoxicity in different cells [10;11;16], while its potency to induce neuroprotection by preconditioning was also much less than isoflurane in this study. Another interesting phenomenon was that preconditioning cells with short exposure of 2.4% isoflurane for 1 hr not only abolished the neurotoxicity of prolonged exposure of 2.4% isoflurane for 24 hr, but also significantly increased cell survival above control level. These results suggested the possible stimulating effects of isoflurane on growth of these cells, for which the mechanisms need further investigation. Our results also suggest that cross preconditioning mechanisms among different inhaled anesthetics may exist as halothane preconditioning also inhibited isoflurane-mediated neurotoxicity.
Reports from recent studies on neurotoxic effects of isoflurane in different cell cultures [16;20;22] and in the developing brains of rodents [8] have raised serious concerns about the safety of anesthesia in surgical patients, particularly in the developing brains of pediatric patients and those patients with Alzheimer's disease. Although the data from this study can not be used directly for suggestions of anesthesia safety in clinical patients, it at least unveiled the double feature of both neuroprotection and neurotoxicity by the commonly used inhaled anesthetic isoflurane. The results of this study call for further investigations in animal models and in patients to determine safe duration and concentration margins for patients to avoid neurotoxicity caused by prolonged exposure to high concentrations of isoflurane. These studies appear to be especially relevant for those patients whose brains may be vulnerable to isoflurane neurotoxicity, as in developing brains and those with Alzheimer's disease.
Acknowledgement
The authors thank Drs. Mark P. Mattson and Sic L. Chan from Laboratory of Neurosciences, National Institute on Aging, Baltimore, Maryland for providing us PC12 cells transfected with Presenilin-1 mutation. We appreciate the useful discussions with Drs. Roderic Eckenhoff and Randall Pittman at the University of Pennsylvania. We would also like to thank Dr. Christopher Ward for his assistance in editing this paper. Supported by National Institute of General Medical Science (NIGMS) K08 grant (1-K08-GM-073224-01, to H.W.), March of Dimes Birth Defects Foundation Research Grant (12-FY05-62, to H.W.) and the Research Fund at the Department of Anesthesiology and Critical Care, University of Pennsylvania (to H.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bickler PE, Fahlman CS. The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth. Analg. 2006;103:419–29. doi: 10.1213/01.ane.0000223671.49376.b2. [DOI] [PubMed] [Google Scholar]
- 2.Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology. 2005;103:532–9. doi: 10.1097/00000542-200509000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 4.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 5.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Guo Q, Furukawa K, Sopher BL, Pham DG, Xie J, Robinson N, Martin GM, Mattson MP. Alzheimer's PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid beta-peptide. Neuroreport. 1996;8:379–83. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- 7.Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP. Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J. Neurosi. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato R, Foex P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can. J. Anaesth. 2002;49:777–791. doi: 10.1007/BF03017409. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Oh E, Im H, Mun J, Yang M, Khim JY, Lee E, Lim SH, Kong MH, Lee M, Sul D. Oxidative damages in the DNA, lipids, and proteins of rats exposed to isofluranes and alcohols. Toxicology. 2006;220:169–178. doi: 10.1016/j.tox.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl HL, Geiger KK, Pannen BH. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102:1147–1157. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Olney JW, Young C, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Do pediatric drugs cause developing neurons to commit suicide? Trends Pharmacol. Sci. 2004;25:135–9. doi: 10.1016/j.tips.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Warner DS. Isoflurane neuroprotection - A passing fantasy, again? Anesthesiology. 2000;92:1226–1228. [PubMed] [Google Scholar]
- 14.Warner DS. Anesthetics provide limited but real protection against acute brain injury. J. Neurosurg. Anesthesiol. 2004;16:303–307. doi: 10.1097/00008506-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Warner DS, McFarlane C, Todd MM, Ludwig P, McAllister AM. Sevoflurane and Halothane Reduce Focal Ischemic Brain-Damage in the Rat - Possible Influence on Thermoregulation. Anesthesiology. 1993;79:985–992. doi: 10.1097/00000542-199311000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Wei H, Leeds P, Chen RW, Wei W, Leng Y, Bredesen DE, Chuang DM. Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. J. Neurochem. 2000;75:81–90. doi: 10.1046/j.1471-4159.2000.0750081.x. [DOI] [PubMed] [Google Scholar]
- 18.Wei HF, Leeds PR, Qian YN, Wei WL, Chen RW, Chuang DM. beta-amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment. Eur. J. Pharmacol. 2000;392:117–123. doi: 10.1016/s0014-2999(00)00127-8. [DOI] [PubMed] [Google Scholar]
- 19.Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS. Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesth. Analg. 2005;101:651–657. doi: 10.1213/01.ane.0000167382.79889.7c. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Xie Z, Dong Y, Maeda U, Moir R, Inouye SK, Culley DJ, Crosby G, Tanzi RE. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J. Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng S, Zuo Z. Isoflurane preconditioning reduces purkinje cell death in an in vitro model of rat cerebellar ischemia. Neuroscience. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]
- 25.Zuo Z, Wang Y, Huang Y. Isoflurane preconditioning protects human neuroblastoma SH-SY5Y cells against in vitro simulated ischemia-reperfusion through the activation of extracellular signal-regulated kinases pathway. Eur. J. Pharmacol. 2006;542:84–91. doi: 10.1016/j.ejphar.2006.05.027. [DOI] [PubMed] [Google Scholar]