Abstract
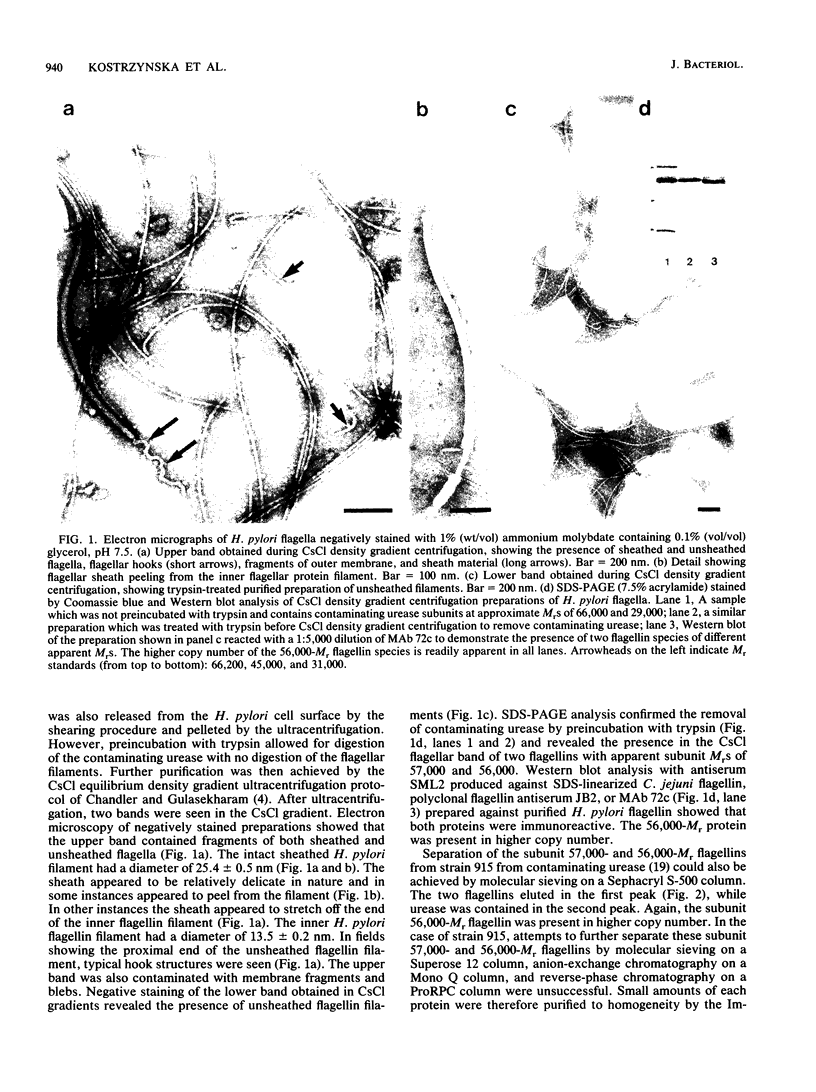
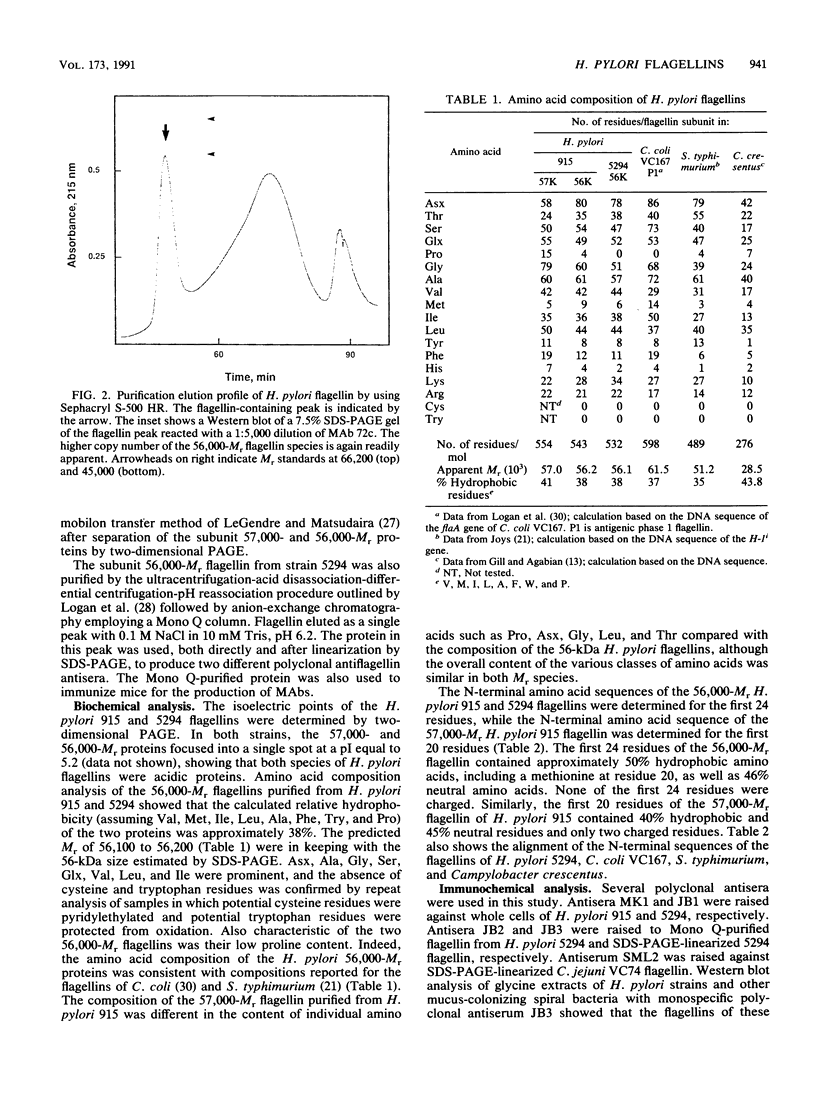
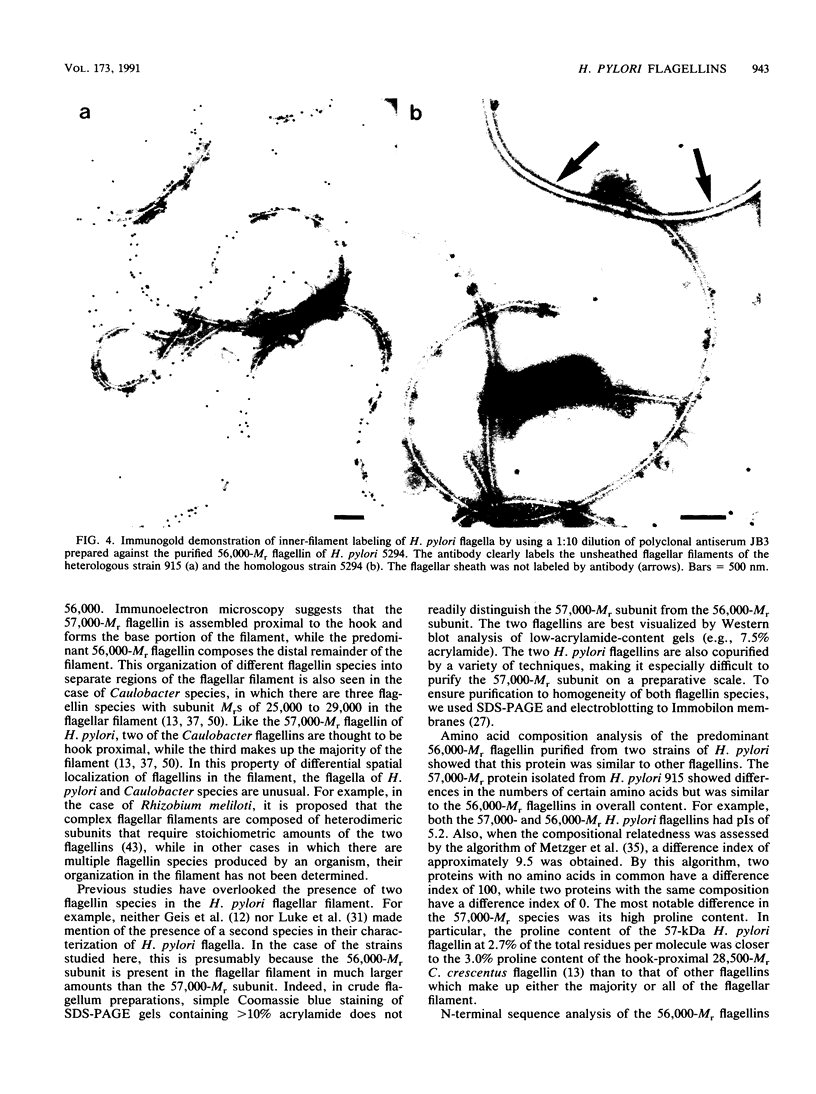
Flagellar filaments were isolated from Helicobacter pylori by shearing, and flagellar proteins were further purified by a variety of techniques, including CsCl density gradient ultracentrifugation, pH 2.0 acid disassociation-neutral pH reassociation, and differential ultracentrifugation followed by molecular sieving with a Sephacryl S-500 column or Mono Q anion-exchange column, and purified to homogeneity by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transfer to an Immobilon membrane. Two flagellin species of pI 5.2 and with apparent subunit molecular weights (Mrs) of 57,000 and 56,000 were obtained. N-terminal amino acid analysis showed that the two H. pylori flagellin species were related to each other and shared sequence similarity with the N-terminal amino acid sequence of Campylobacter coli, Bacillus, Salmonella, and Caulobacter flagellins. Analysis of the amino acid composition of the predominant 56,000-Mr flagellin species isolated from two strains showed that it was comparable to the flagellins of other species. The minor 57,000-Mr flagellin species contained a higher content of proline. Immunoelectron microscopic studies with polyclonal monospecific H. pylori antiflagellin antiserum and monoclonal antibody (MAb) 72c showed that the two different-Mr flagellin species were located in different regions of the assembled flagellar filament. The minor 57,000-Mr species was located proximal to the hook, and the major 56,000-Mr flagellin composed the remainder of the filament. Western immunoblot analysis with polyclonal rabbit antisera raised against H. pylori or Campylobacter jejuni flagellins and MAb 72c showed that the 56,000-Mr flagellin carried sequences antigenetically cross-reactive with the 57,000-Mr H. pylori flagellin and the flagellins of Campylobacter species. This antigenic cross-reactivity did not extend to the flagellins of other gram-negative bacteria. The 56,000-Mr flagellin also carried H. pylori-specific sequences recognized by two additional MAbs. The epitopes for these MAbs were not surface exposed on the assembled inner flagellar filament of H. pylori but were readily detected by immunodot blot assay of sodium dodecyl sulfate-lysed cells of H. pylori, suggesting that this serological test could be a useful addition to those currently employed in the rapid identification of this important pathogen.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Epidemiology and pathophysiology of Campylobacter pylori infections. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 1):S99–106. doi: 10.1093/clinids/12.supplement_1.s99. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Chandler H. M., Gulasekharam J. The protective antigen of a highly immunogenic strain of clostridium chauvoei including an evaluation of its flagella as a protective antigen. J Gen Microbiol. 1974 Sep;84(1):128–134. doi: 10.1099/00221287-84-1-128. [DOI] [PubMed] [Google Scholar]
- Cheng S. C., Sanderson C. R., Waters T. E., Goodwin C. S. Campylobacter pyloridis in patients with gastric carcinoma. Med J Aust. 1987 Aug 17;147(4):202–203. doi: 10.5694/j.1326-5377.1987.tb133380.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- DeLange R. J., Chang J. Y., Shaper J. H., Glazer A. N. Amino acid sequence of flagellin of Bacillus subtilis 168. III. Tryptic peptides, N-bromosuccinimide peptides, and the complete amino acid sequence. J Biol Chem. 1976 Feb 10;251(3):705–711. [PubMed] [Google Scholar]
- Dunn B. E., Perez-Perez G. I., Blaser M. J. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter pylori proteins. Infect Immun. 1989 Jun;57(6):1825–1833. doi: 10.1128/iai.57.6.1825-1833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst J. A., Perry J. W. Demonstration of lipopolysaccharide on sheathed flagella of Vibrio cholerae O:1 by protein A-gold immunoelectron microscopy. J Bacteriol. 1988 Apr;170(4):1488–1494. doi: 10.1128/jb.170.4.1488-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis G., Leying H., Suerbaum S., Mai U., Opferkuch W. Ultrastructure and chemical analysis of Campylobacter pylori flagella. J Clin Microbiol. 1989 Mar;27(3):436–441. doi: 10.1128/jcm.27.3.436-441.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P. R., Agabian N. The nucleotide sequence of the Mr = 28,500 flagellin gene of Caulobacter crescentus. J Biol Chem. 1983 Jun 25;258(12):7395–7401. [PubMed] [Google Scholar]
- Goodwin C. S., McCulloch R. K., Armstrong J. A., Wee S. H. Unusual cellular fatty acids and distinctive ultrastructure in a new spiral bacterium (Campylobacter pyloridis) from the human gastric mucosa. J Med Microbiol. 1985 Apr;19(2):257–267. doi: 10.1099/00222615-19-2-257. [DOI] [PubMed] [Google Scholar]
- Guerry P., Logan S. M., Thornton S., Trust T. J. Genomic organization and expression of Campylobacter flagellin genes. J Bacteriol. 1990 Apr;172(4):1853–1860. doi: 10.1128/jb.172.4.1853-1860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. A., Logan S. M., Guerry P., Trust T. J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987 Nov;169(11):5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell S. L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986 Apr;153(4):658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Hu L. T., Mobley H. L. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun. 1990 Apr;58(4):992–998. doi: 10.1128/iai.58.4.992-998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S. J., Liu W. Z., Zhang D. Z., Shi Y., Xiao S. D., Zhang Z. H., Lu D. Y. Campylobacter-like organisms in chronic gastritis, peptic ulcer, and gastric carcinoma. Scand J Gastroenterol. 1987 Jun;22(5):553–558. doi: 10.3109/00365528708991897. [DOI] [PubMed] [Google Scholar]
- Joys T. M., Rankis V. The primary structure of the phase-1 flagellar protein of Salmonella typhimurium. I. The tryptic peptides. J Biol Chem. 1972 Aug 25;247(16):5180–5193. [PubMed] [Google Scholar]
- Krakowka S., Morgan D. R., Kraft W. G., Leunk R. D. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun. 1987 Nov;55(11):2789–2796. doi: 10.1128/iai.55.11.2789-2796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert J. R., Borromeo M., Pinkard K. J., Turner H., Chapman C. B., Smith M. L. Colonization of gnotobiotic piglets with Campylobacter Pyloridis--an animal model? J Infect Dis. 1987 Jun;155(6):1344–1344. doi: 10.1093/infdis/155.6.1344. [DOI] [PubMed] [Google Scholar]
- LeGendre N., Matsudaira P. Direct protein microsequencing from Immobilon-P Transfer Membrane. Biotechniques. 1988 Feb;6(2):154–159. [PubMed] [Google Scholar]
- Logan S. M., Harris L. A., Trust T. J. Isolation and characterization of Campylobacter flagellins. J Bacteriol. 1987 Nov;169(11):5072–5077. doi: 10.1128/jb.169.11.5072-5077.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J., Guerry P. Evidence for posttranslational modification and gene duplication of Campylobacter flagellin. J Bacteriol. 1989 Jun;171(6):3031–3038. doi: 10.1128/jb.171.6.3031-3038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Location of epitopes on Campylobacter jejuni flagella. J Bacteriol. 1986 Nov;168(2):739–745. doi: 10.1128/jb.168.2.739-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke C. J., Kubiak E., Cockayne A., Elliott T. S., Penn C. W. Identification of flagellar and associated polypeptides of Helicobacter (formerly Campylobacter) pylori. FEMS Microbiol Lett. 1990 Sep 1;59(1-2):225–230. doi: 10.1016/0378-1097(90)90061-t. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Armstrong J. A., McGechie D. B., Glancy R. J. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust. 1985 Apr 15;142(8):436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Campylobacter pylori: its link to gastritis and peptic ulcer disease. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 1):S87–S93. doi: 10.1093/clinids/12.supplement_1.s87. [DOI] [PubMed] [Google Scholar]
- Marshall T., Williams K. M., Vesterberg O. Two-dimensional electrophoresis of proteins in human serum: improved resolution by use of narrow pH gradients and prolonged electrophoresis. Clin Chem. 1984 Dec;30(12 Pt 1):2008–2013. [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- Mills S. D., Kurjanczyk L. A., Penner J. L. Identification of an antigen common to different species of the genus Campylobacter. J Clin Microbiol. 1988 Jul;26(7):1411–1413. doi: 10.1128/jcm.26.7.1411-1413.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich S. A., Newton A. Promoter mapping and cell cycle regulation of flagellin gene transcription in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1142–1146. doi: 10.1073/pnas.84.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A., Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987 Mar;82(3):192–199. [PubMed] [Google Scholar]
- Newell D. G. Identification of the outer membrane proteins of Campylobacter pyloridis and antigenic cross-reactivity between C. pyloridis and C. jejuni. J Gen Microbiol. 1987 Jan;133(1):163–170. doi: 10.1099/00221287-133-1-163. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. W., Pinder M., Roelants G. E., Kar S. K., Lundin L. B., Mayor-Withey K. S., Hwett R. S. Methods for derivation and detection of anti-parasite monoclonal antibodies. J Immunol Methods. 1980;34(2):141–154. doi: 10.1016/0022-1759(80)90168-4. [DOI] [PubMed] [Google Scholar]
- Penner J. L. The genus Campylobacter: a decade of progress. Clin Microbiol Rev. 1988 Apr;1(2):157–172. doi: 10.1128/cmr.1.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleier E., Schmitt R. Identification and sequence analysis of two related flagellin genes in Rhizobium meliloti. J Bacteriol. 1989 Mar;171(3):1467–1475. doi: 10.1128/jb.171.3.1467-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E. F., Dannelly H. K., Malloy P. J., Reeves H. C. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem. 1987 Dec;167(2):290–294. doi: 10.1016/0003-2697(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., Zoltowska B., Trust T. J., Lane D. J., Olsen G. J., Pace N. R., Stahl D. A. Campylobacter pylori, the spiral bacterium associated with human gastritis, is not a true Campylobacter sp. J Bacteriol. 1987 May;169(5):2137–2141. doi: 10.1128/jb.169.5.2137-2141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow L. S., Rittenberg S. C. Isolation and composition of sheathed flagella from Bdellovibrio bacteriovorus 109J. J Bacteriol. 1985 Sep;163(3):1047–1054. doi: 10.1128/jb.163.3.1047-1054.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. A., Logan S. M., Trust T. J., Guerry P. Polynucleotide sequence relationships among flagellin genes of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1990 Aug;58(8):2686–2689. doi: 10.1128/iai.58.8.2686-2689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Weissborn A., Steinmann H. M., Shapiro L. Characterization of the proteins of the Caulobacter crescentus flagellar filament. Peptide analysis and filament organization. J Biol Chem. 1982 Feb 25;257(4):2066–2074. [PubMed] [Google Scholar]
- Wyatt J. I. The role of Campylobacter pylori in the pathogenesis of peptic ulcer disease. Scand J Gastroenterol Suppl. 1989;157:7–22. doi: 10.3109/00365528909091044. [DOI] [PubMed] [Google Scholar]
- Yokote Y., Arai K. M., Akahane K. Recovery of tryptophan from 25-minute acid hydrolysates of protein. Anal Biochem. 1986 Feb 1;152(2):245–249. doi: 10.1016/0003-2697(86)90405-7. [DOI] [PubMed] [Google Scholar]








