Abstract
Patients with newly diagnosed non-small cell lung cancer (NSCLC) need accurate tumor staging in order to direct appropriate therapy and establish prognosis; the tumor is usually staged using the TNM system. The major imaging modalities currently used for staging this disease are thoracic computed tomography (CT) (including the adrenal glands) and whole body fluorodeoxyglucose (FDG)-positron emission tomography (PET) scanning. CT is generally most useful in evaluating the T stage, i.e. local spread of the neoplasm, whereas PET is most helpful in assessing the N and M stages, i.e. regional and distant tumor spread, respectively. Integrated CT-PET imaging adds information compared to the use of either modality alone. PET findings frequently lead to upstaging the disease and thus prevent unindicated surgeries. Magnetic resonance imaging (MRI) is helpful in evaluating local extent of disease in patients with superior sulcus tumors and possible brachial plexus involvement. Staging accuracy using any of these imaging techniques is imperfect; therefore, pathologic confirmation of positive findings is recommended, whenever possible, before denying a patient potentially curative therapy.
Keywords: Non-small cell lung cancer, neoplasm staging, computed tomography, FDG-PET
Introduction
Initial staging of extent of disease is important in patients with newly diagnosed non-small cell lung cancer (NSCLC), in order to direct appropriate therapy and establish prognosis. Patients with disease localized to a pulmonary lobe, with or without hilar lymph node disease, are usually treated with surgical resection, although radiation therapy may be used in patients who are medically unfit for surgery. If the disease has spread to the mediastinum, treatment is variable. Tumor spread to distant sites is generally treated with chemotherapy. Various imaging modalities may be used to enable tumor staging. Computed tomography (CT) and fluorodeoxyglucose (FDG)-positron emission tomography (PET) scanning are the workhorses for staging; other modalities, such as magnetic resonance imaging (MRI), ultrasonography, bone radiography, bone scintigraphy, and endoscopic ultrasound may be used for specific problem solving and/or to facilitate tissue biopsy. NSCLC is generally staged using the TNM system of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) (Table 1) [1–3]. Definitions according to the current TNM system, published in 2002, are listed below.
Table 1.
Stage groupings (adapted from AJCC[1])
| Stage IA | T1 | N0 | M0 |
| Stage IB | T2 | N0 | M0 |
| Stage IIA | T1 | N1 | M0 |
| Stage IIB | T2 | N1 | M0 |
| T3 | N0 | M0 | |
| Stage IIIA | T3 | N1 | M0 |
| T1–3 | N2 | M0 | |
| Stage IIIB | T4 | Any N | M0 |
| T1–3 | N3 | M0 | |
| Stage IV | Any T | Any N | M1 |
Primary tumor staging (T Stage)
T1 tumors are small (≤3 cm) and surrounded by lung or visceral pleura (Fig. 1). These lesions may involve a lobar or more peripheral bronchus, but they may not involve a mainstem bronchus.
T2 tumors are larger than 3 cm in diameter or they involve a distal mainstem bronchus (>2 cm from the carina) and/or they invade the visceral pleura (Fig. 2). T2 lesions may have post-obstructive atelectasis or pneumonia that involves less than an entire lung.
T3 lesions may be of any size if they directly invade any of the following: chest wall (including superior sulcus), diaphragm, mediastinal pleura, parietal pericardium, or proximal mainstem bronchus (<2 cm from the carina) (Fig. 3). There may be post-obstructive atelectasis or pneumonia that involves an entire lung.
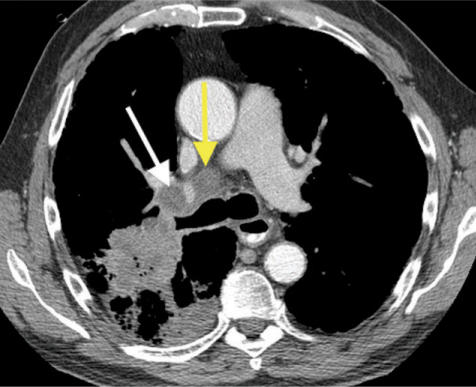
T4 tumors may be of any size if they directly invade any of the following: mediastinum, heart, great vessels, trachea, esophagus, vertebral body, or carina (Fig. 4). This category includes those tumors with one or more satellite tumor nodules within the same lobe of the lung and/or with a malignant pleural or pericardial effusion.
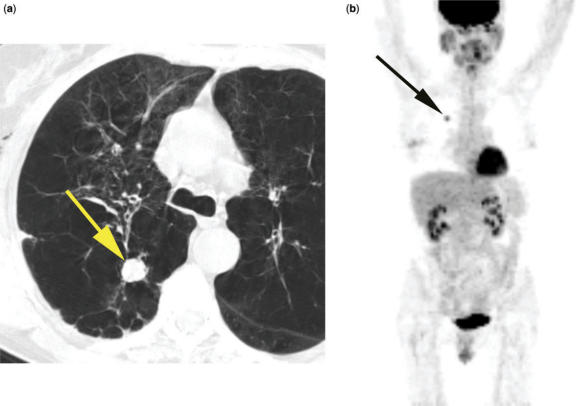
Figure 1.
T1N0M0 adenocarcinoma of lung (arrow) on CT (a) and PET (b). Small tumor surrounded by lung.
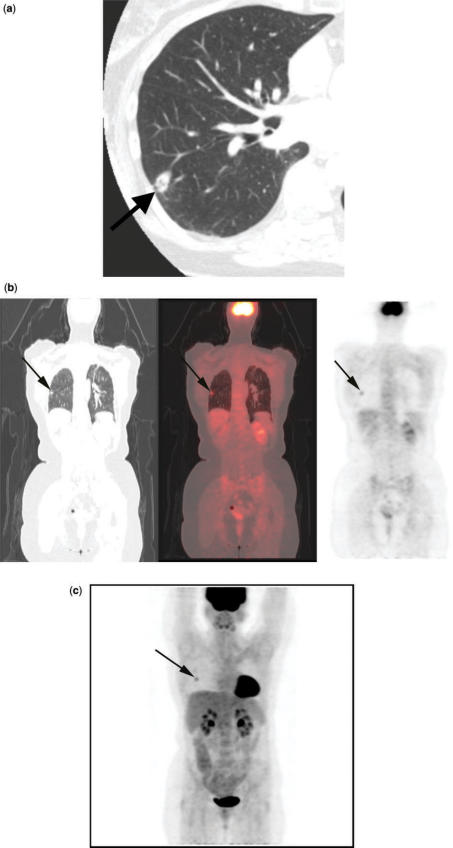
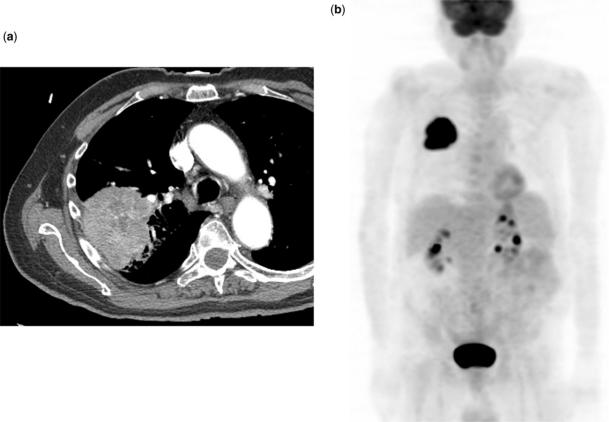
Figure 2.
T2N0M0 squamous cell lung cancer. CT (a) shows a small, cavitary nodule with stranding extending to the pleural surface (arrow). Integrated coronal CT-PET images (b) and 3D PET image (c) demonstrate the FDG avid nodule (arrow). Histopathological examination of the resected specimen revealed visceral pleural tumor invasion.
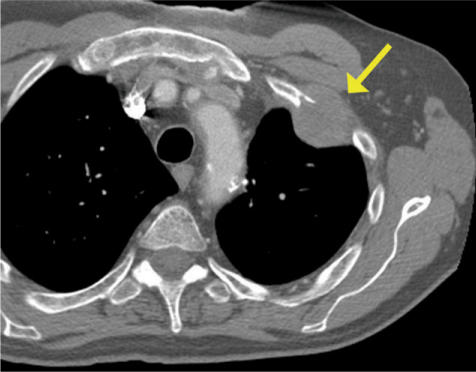
Figure 3.
T3 adenocarcinoma invading the chest wall. Rib destruction and frank, abnormal soft tissue in the chest wall on CT (arrow).
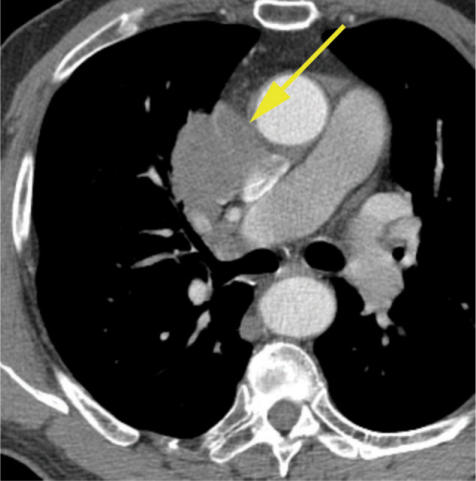
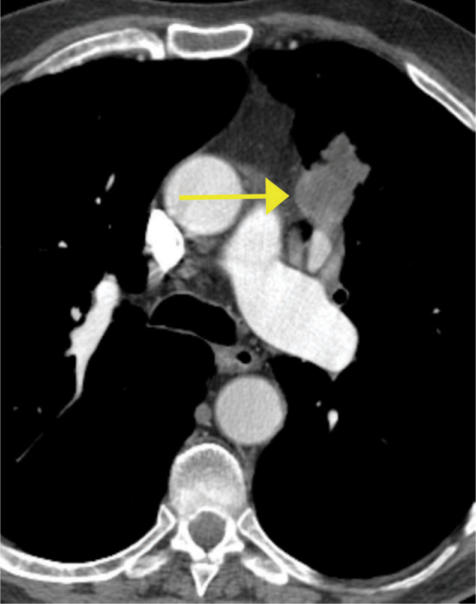
Figure 4.
T4 squamous cell lung cancer with gross, direct mediastinal invasion (arrow). The tumor abuts the superior vena cava and the aorta and therefore may be invading these structures.
Evaluating T status
Among the available imaging modalities, T status is generally best evaluated using CT scanning. CT is excellent for establishing tumor size and relationship to the central airways, and for detection of additional tumor nodules. However, it has limitations in evaluating for tumor invasion into adjacent structures.
Chest wall invasion
CT has shown somewhat disparate results in assessing for chest wall invasion by tumor, with sensitivity ranging from 38% to 87% and specificity from 40% to 90%[4–6]. Signs of invasion may include pleural thickening, loss of the extrapleural fat plane, obtuse angle between mass and chest wall, and greater than 3 cm of contact between mass and chest wall (Fig. 5). However, the only reliable criterion for diagnosing chest wall invasion with routine CT is definite bone destruction, with or without tumor mass extending into the chest wall (Fig. 3). Some investigators have employed induced pneumothorax CT in order to increase the accuracy of CT in diagnosing chest wall and mediastinal pleural invasion. False positive cases of invasion may occur due to difficulty in introducing air into some regions of the pleural space, particularly near the hila. Other techniques for diagnosing chest wall invasion rely upon lack of relative movement between the chest wall and the adjacent tumor during respiration. Some investigations have used inspiratory/expiratory CT, ultrasonography, and cine-MR with during deep respirations to evaluate this feature, with moderate success. Both pneumothorax CT and inspiratory/expiratory CT may show false positives due to benign inflammatory pleural adhesions.
Figure 5.
Right upper lobe adenocarcinoma showing broad contact with the pleural surface. The findings are indeterminate for chest wall invasion at CT (a) and PET (b).
MR shows similar accuracy compared to CT in assessing for chest wall invasion. MR findings of chest wall invasion include disruption of the normally high signal intensity extrapleural fat stripe by moderate intensity soft tissue on T1-weighted images or abnormal, high signal intensity tissue on T2 weighted images. In the past, MR has been considered superior to CT in assessing for superior sulcus invasion, primarily due to its ability to image in any plane; however, the current multiplanar reconstruction capabilities of CT yield similar results. On the other hand, MR is still the modality of choice in evaluating for possible tumor involvement of the brachial plexus or spinal canal (Fig. 6). PET scanning is generally not useful for diagnosing chest wall invasion due to its poor spatial resolution (Fig. 5).
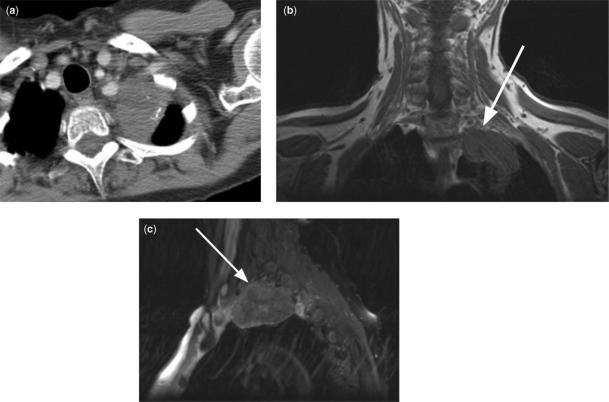
Figure 6.
Left apical NSCLC in a 61-year-old woman with left shoulder pain. On axial CT (a), the tumor is indeterminate for superior sulcus invasion. Coronal T1 weighted MR (b) and para-sagittal T2 weighted MR (c) images show tumor invasion of the inferior portions of the brachial plexus (arrow) (T3 tumor).
It should be noted that chest wall invasion does not preclude surgical resection, because the surgeon may perform en bloc resection and chest wall reconstruction. However, this procedure is associated with increased operative morbidity and mortality. Furthermore, chest wall resection is contraindicated when accompanied by mediastinal lymph node metastases due to the poor prognosis of such patients (7% 5-year survival following surgical resection)[7,8].
Mediastinal invasion
Although invasion of the mediastinum falls into the T4 category in the TNM staging classification, minimal invasion of fat only (without invasion of vascular or other structures) is generally considered resectable by many surgeons. Therefore it is not usually necessary to preoperatively diagnose minimal mediastinal fat invasion. On the other hand, a reliable diagnosis of gross invasion of mediastinal fat or invasion of mediastinal vessels, trachea, esophagus, and/or vertebral body would preclude surgical resection. CT diagnosis of minimal mediastinal fat is generally unreliable[4,6,9] (Fig. 7). With regard to mediastinal structure invasion, it is often difficult to differentiate between tumor abutment of a structure and actual invasion (Fig. 4). Therefore, a patient should not be denied surgery based on unproven CT findings. Gross mediastinal fat invasion may be proved via mediastinoscopy or transtracheal Wang needle biopsy, if the location is accessible using these techniques. Findings suggestive of central tracheobronchial invasion at CT are usually further evaluated using bronchoscopy. Sometimes secondary signs are useful in diagnosing mediastinal invasion; for example, vocal cord deviation and hoarseness in a patient with a left lung cancer suggests mediastinal tumor invasion involving the recurrent laryngeal nerve.
Figure 7.
Left upper lobe squamous cell cancer showing a broad, convex margin with the mediastinum at CT (arrow); there was no mediastinal or pleural invasion at surgery or pathology (T2 tumor).
MRI has the same limitations as CT in evaluating for mediastinal invasion, and it shows similar accuracy in this setting. It has been suggested that transesophageal echocardiography (TEE) may be a useful technique to assess for aortic invasion, surpassing CT accuracy in this regard[10].
Pleural invasion
A pleural effusion in a patient with lung cancer may be malignant, caused by pleural metastases, or it may be benign, particularly in a patient with postobstructive pneumonia. The CT hallmark for a malignant effusion is soft tissue nodularity along the pleural surfaces, accompanying the effusion, although this finding is not always present. Pleural nodularity and/or fissural thickening are suggestive of pleural metastases, even in the absence of pleural effusion. FDG-PET scanning has shown good results in distinguishing benign from malignant pleural effusions, with sensitivity figures ranging from 70 to 95% and specificity from 64 to 94%[11–14]. Pleural tumor dissemination is considered unresectable.
Regional lymph node staging (N Stage)
N1 Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes, and intrapulmonary nodes involved by direct extension from the primary tumor (Figs. 8 and 9)
N2 Metastasis in ipsilateral mediastinal and/or subcarinal lymph nodes (Figs. 9–11)
N3 Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene or supraclavicular lymph nodes (Figs. 10 and 11).
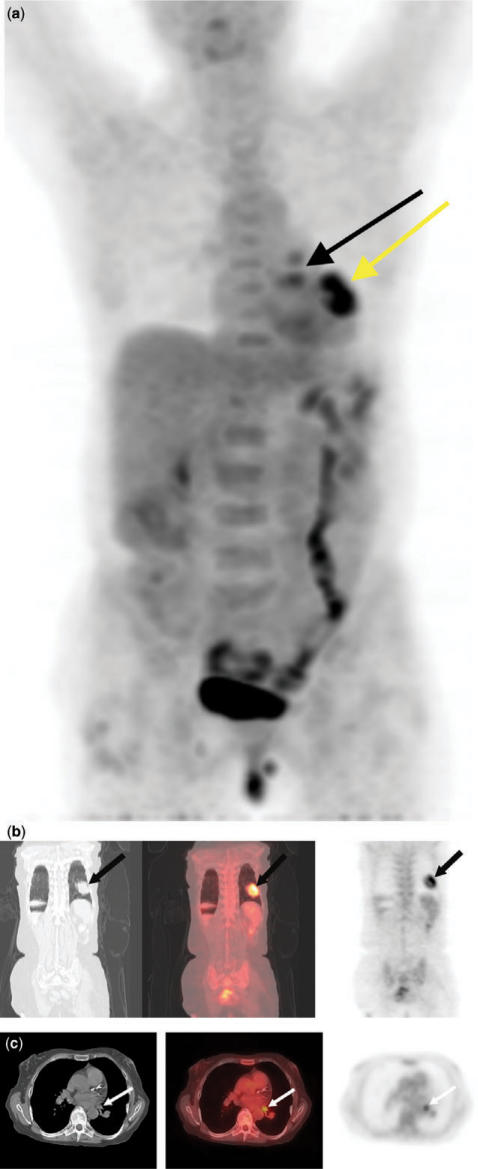
Figure 8.
T2N1 left lower lobe squamous cell cancer with hilar lymph node metastases. 3D PET image (a) shows uptake in the lung (yellow arrow) and in the region of the left hilum (black arrow). Coronal (b) and axial (c) integrated CT-PET images confirm uptake in the lung mass and a hilar lymph node, respectively (arrows).
Figure 9.
Right upper lobe squamous cell cancer with post-obstructive pneumonia. CT shows enlarged lymph nodes in the right hilar (white arrow) and tracheobronchial (yellow arrow) regions, suggesting N1 and N2 disease, respectively. Alternatively, these could represent reactive lymph nodes, draining the right upper lobe pneumonia.
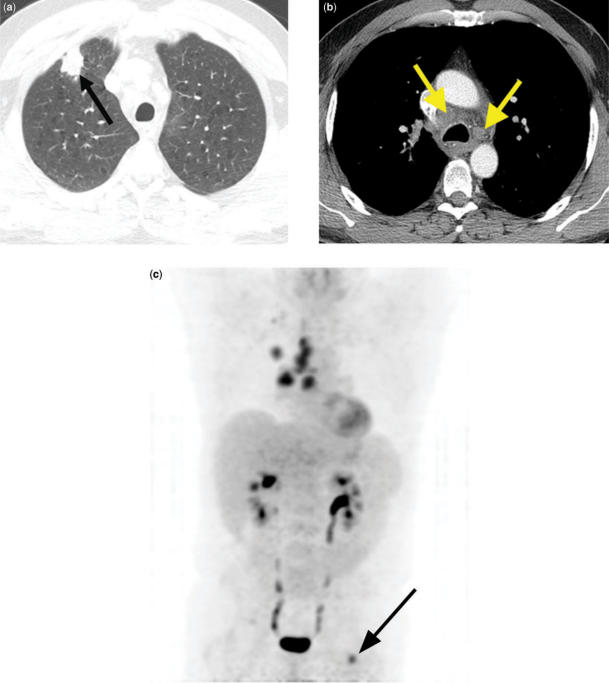
Figure 10.
Right upper lobe squamous cell cancer. Enlarged ipsilateral and contralateral mediastinal lymph nodes at CT (a) (arrows) and bilateral abnormal uptake on a 3D PET image (b) suggest N2 and N3 disease. Integrated coronal CT-PET images (c,d) confirm abnormal uptake within the lung mass (c), as well as bilateral mediastinal lymph nodes (black arrows), a left supraclavicular lymph node (yellow arrow), and a left axillary lymph node (red arrow) (d). Axillary lymph node metastasis represents M1 disease.
Figure 11.
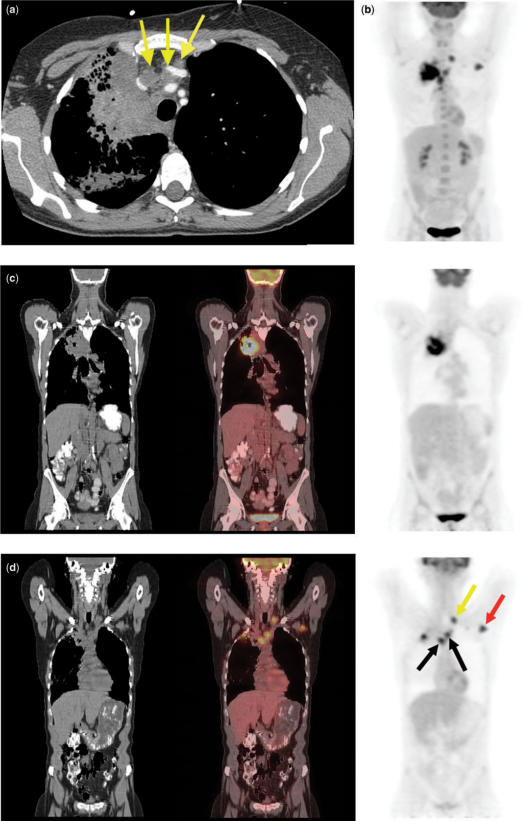
Right upper lobe adenocarcinoma. CT shows a right upper lobe nodule (a) (arrow) and enlarged ipsilateral and contralateral mediastinal lymph nodes (b) (arrows). 3D PET image (c) confirms abnormal radiotracer uptake within these areas, consistent with N2 and N3 disease. In addition, PET reveals a previously unsuspected distant metastasis in the left femur (M1 disease) (arrow).
Evaluating N status
The presence of metastatic disease in hilar lymph nodes (N1 disease) affects patient prognosis, but does not generally affect resectability. Moreover, the presence or absence of hilar nodal metastases does not reliably predict the status of the mediastinal lymph nodes. Tumors with metastatic disease to ipsilateral mediastinal nodes (N2 disease) are potentially resectable, generally after neoadjuvant chemotherapy and/or radiotherapy, as long as the nodes are not numerous and/or bulky[15]. Contralateral hilar or mediastinal lymph node disease or metastases to any scalene or supraclavicular lymph nodes (N3 disease) precludes surgery. It has been noted that the presence of mediastinal nodal disease indicates aggressive tumor biology, suggesting the presence of distant disease and poor survival[15].
CT criteria for lymph node metastases theoretically include morphological features such as nodal attenuation and margination. In practice, however, these features are usually unhelpful, and nodal enlargement (>1 cm in short axis diameter) is the only currently useful diagnostic criterion. Disparate results have been reported regarding the accuracy of CT in diagnosing hilar and mediastinal lymph node metastases. Many of the more recent studies have shown low accuracy, resulting from both poor sensitivity (48–65%) and poor specificity (53–79%)[4,9,16–19]. Low sensitivity in some studies was attributed to the high frequency of microscopic metastases within normal sized nodes. Low specificity arose from the frequent occurrence of enlarged, hyperplastic nodes, particularly in patients with postobstructive pneumonitis[16] (Fig. 9).
MRI shows similar limitations compared to CT in evaluating for N1 and N2 disease, and reported diagnostic accuracies are similar. On the other hand, FDG-PET scanning is clearly superior to either CT or MRI in this setting. PET sensitivity and specificity are approximately 80% and 90%, respectively, in diagnosing mediastinal lymph node metastases[20–23], and integrated CT-PET imaging appears to offer diagnostic advantages compared to PET alone[24]. PET sensitivity increases and specificity decreases for enlarged lymph nodes, whereas sensitivity decreases and specificity increases for small lymph nodes[25]. The major pitfalls with PET include false positives due to inflammatory lymph nodes (accounting for poor specificity in enlarged lymph nodes) and false negatives due to small metastases that are below the spatial limits of resolution (accounting for poor sensitivity for small lymph nodes).
It is generally agreed that all patients with enlarged mediastinal lymph nodes at CT (and without a negative PET scan) and all patients with abnormal mediastinal lymph nodes at PET scanning need lymph node biopsy for confirmation; therapy should not be planned based upon unproven, positive imaging findings alone. Lymph node locations on CT have been mapped to American Thoracic Society (ATS) lymph node stations in a recently published CT atlas[26]; reference to such standard locations may aid in directing preoperative lymph node sampling via transbronchoscopic Wang needle biopsy, mediastinoscopy, TEE, or video assisted thoracoscopic surgery (VATS), according to the location of the lymph nodes.
Unlike patients with bulky mediastinal lymph node metastases, those with microscopic metastases in normal sized lymph nodes may benefit from surgical resection[18]. Therefore, many surgeons believe that a negative CT scan (or a negative CT-PET scan) obviates the need for preoperative lymph node sampling, and these patients should go directly to thoracotomy. The exception occurs in patients with adenocarcinomas or T3 tumors, including Pancoast tumors; mediastinoscopy may be indicated in such patients because the concomitant presence of mediastinal nodal metastases portends a poor prognosis, and therefore such patients are not usually felt to be surgical candidates[15,27].
Distant metastasis staging (M status)
M0 No distant metastasis
M1 distant metastasis present (includes separate metastatic tumor nodule(s) in the ipsilateral non-primary tumor lobe of the lung) (Figs. 10 and 11).
Approximately 18–36% of patients with a new diagnosis of NSCLC have distant metastatic disease[28–31]. Patients with adenocarcinoma appear to be at significantly greater risk for metastases outside the thorax than those with squamous cell cancer. Brain, bone, liver, and adrenal glands are the most common sites of disease, in decreasing order of frequency. Brain metastases often occur as an isolated finding. Some investigators suggest obtaining brain CTs for all patients with adenocarcinoma and large cell carcinoma, as well as for patients with a combination of squamous cell cancer and neurologic symptoms. Unlike brain metastases, isolated liver metastases are uncommon, and therefore the incremental yield of abdominal CT over chest CT is quite small[32].
Despite the high prevalence of adrenal metastases from bronchogenic carcinoma, approximately two-thirds of adrenal masses in patients with NSCLC actually represent adenomas, rather than metastases. Unfortunately, there is significant overlap in the appearance of adrenal metastases and benign adenomas on routine, contrast enhanced CT. Therefore detection of an adrenal mass on such a study requires further workup. Considerable work has recently been done using non-contrast CT, delayed enhanced CT, and MR in evaluating adrenal masses[33–37], and there is data suggesting that PET scanning is also useful in this setting[38–41]. With these techniques, it is often possible to definitively diagnose benign adrenal cortical adenomas without biopsy. If these imaging studies suggest the presence of a metastasis, biopsy proof is generally required before altering therapy.
FDG-PET scanning has been reported to show previously unsuspected distant metastases in approximately 10–20% of patients, with high accuracy[42–45]. These metastases are usually found in the abdomen, and occasionally in lung or bone (Fig. 11). On the other hand, PET is often helpful in excluding metastatic disease in suspicious areas found on CT. PET may show false positive lesions, particularly in vessels, GI tract, muscles and fat, although errors are reduced when co-registering CT images with PET data. False negative lesions may also occur, primarily due to the spatial limits of resolution of the technique.
Future staging system modifications
The International Association for the Study of Lung Cancer recently published a series of articles outlining recommendations for amendments to the TNM staging system based on data from more than 100,000 patients in its Lung Cancer Staging Project[46–50]. These amendments, which will probably be reflected in the next official version of the TNM classification system (due to be published in 2009), include the following: the T1 and T2 categories are broken down by tumor diameter (T1a, ≤2 cm; T1b, >2–3 cm; T2a, >3–5 cm; T2b, >5–7 cm). In addition, T3 includes tumors >7 cm in diameter. A satellite nodule (or nodules) in the same lobe of the lung as the primary tumor falls into the T3 category; if the satellite nodule is in a different, ipsilateral lobe, this represents T4 disease; and if it is in a contralateral lobe, this presents M1a disease. The N classification has no changes. The M category has been subdivided into M1a and M1b. M1a includes patients with distant metastatic disease confined to the lung and pleura, such as malignant pleural nodules, malignant pleural or pericardial effusion, or separate tumor nodule(s) in a contralateral lobe. The M1b category includes distant metastases outside of the lung and pleura. The stage groupings have also changed somewhat to better align the classifications with prognosis and treatment[46].
Conclusion
CT offers useful information in the staging work-up of patients with NSCLC. However, CT staging accuracy is imperfect, and it is important to know the capabilities and limitations of the technique in order to appropriately triage patients for therapy or further diagnostic testing. The addition of FDG-PET scanning to the conventional work up has been reported to change management in 20–30% of patients, mostly by upstaging the disease[51,52]. A recent randomized control trial reported that adding PET reduced futile thoracotomies by half[53]. PET is felt to be a cost effective technique, primarily due to this significant reduction in the number of unindicated surgeries[27,53]. Current NCCN (National Comprehensive Cancer Network, USA) guidelines advocate PET scanning for all patients with a new diagnosis of NSCLC, except for clinical Stage IV patients with known, disseminated distant metastatic disease. However, the NCCN does recommend pathologic confirmation of positive PET findings. Similar guidelines have been put forth by the National Institute for Clinical Excellence in the United Kingdom[54].
References
- [1].AJCC. AJCC cancer staging handbook. New York: Springer-Verlag; 2002. [Google Scholar]
- [2].Mountain C. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–17. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- [3].Mountain CF. Staging classification of lung cancer. A critical evaluation. Clin Chest Med. 2002;23:103–21. doi: 10.1016/s0272-5231(03)00063-7. [DOI] [PubMed] [Google Scholar]
- [4].Gdeedo A, VanSchil P, Corthouts B, VanMieghem F, VanMeerbeeck J, VanMarck E. Comparison of imaging TNM [(i)TNM] and pathological TNM [(p)TNM] in staging of bronchogenic carcinoma. Eur J Cardiothorac Surg. 1997;12:224–7. doi: 10.1016/s1010-7940(97)00084-5. [DOI] [PubMed] [Google Scholar]
- [5].Ratto G, Piacenza G, Frola C. Chest wall involvement by lung cancer: computed tomographic detection and results of operation. Ann Thorac Surg. 1991;51:182–8. doi: 10.1016/0003-4975(91)90778-o. [DOI] [PubMed] [Google Scholar]
- [6].Venuta F, Rendina E, Ciriaco P. Computed tomography for preoperative assessment of T3 and T4 bronchogenic carcinoma. Eur J Cardiothorac Surg. 1992;6:238–41. doi: 10.1016/1010-7940(92)90104-6. [DOI] [PubMed] [Google Scholar]
- [7].Paone J, Spees E, Newton C, Lilemoe K, Kieffer R, Gadacz T. An appraisal of en bloc resection of peripheral bronchogenic carcinoma involving the thoracic wall. Chest. 1982;81:203–7. doi: 10.1378/chest.81.2.203. [DOI] [PubMed] [Google Scholar]
- [8].Piehler J, Pairolero P, Weiland L, Offord K, Payne W. Bronchogenic carcinoma with chest wall invasion: factors affecting survival following en bloc resection. Ann Thorac Surg. 1982;34:684–91. doi: 10.1016/s0003-4975(10)60909-5. [DOI] [PubMed] [Google Scholar]
- [9].Webb W, Gatsonis C, Zerhouni E, et al. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the radiologic diagnostic oncology group. Radiology. 1991;178:705–13. doi: 10.1148/radiology.178.3.1847239. [DOI] [PubMed] [Google Scholar]
- [10].Schroder C, Schonhofer B, Vogel B. Transesophageal echographic determination of aortic invasion by lung cancer. Chest. 2005;127:438–42. doi: 10.1378/chest.127.2.438. [DOI] [PubMed] [Google Scholar]
- [11].Gupta NC, Rogers JS, Graeber GM, et al. Clinical role of F-18 fluorodeoxyglucose positron emission tomography imaging in patients with lung cancer and suspected malignant pleural effusion. Chest. 2002;122:1918–24. doi: 10.1378/chest.122.6.1918. [DOI] [PubMed] [Google Scholar]
- [12].Erasmus JJ, McAdams HP, Rossi SE, Goodman PC, Coleman RE, Patz EF. FDG PET of pleural effusions in patients with non-small cell lung cancer. AJR. 2000;175:245–9. doi: 10.2214/ajr.175.1.1750245. [DOI] [PubMed] [Google Scholar]
- [13].Duysinx BC, Larock MP, Nguyen D, et al. 18F-FDG PET imaging in assessing exudative pleural effusions. Nucl Med Commun. 2006;27:971–6. doi: 10.1097/01.mnm.0000243366.96012.c0. [DOI] [PubMed] [Google Scholar]
- [14].Toaff JS, Metser U, Gottfried M, et al. Differentiation between malignant and benign pleural effusion in patients with extra-pleural primary malignancies: assessment with positron emission tomography-computed tomography. Invest Radiol. 2005;40:204–9. doi: 10.1097/01.rli.0000154217.71461.b4. [DOI] [PubMed] [Google Scholar]
- [15].Patel V, Shrager JB. Which patients with stage III non-small cell lung cancer should undergo surgical resection? Oncologist. 2005;10:335–44. doi: 10.1634/theoncologist.10-5-335. [DOI] [PubMed] [Google Scholar]
- [16].McLoud T, Bourgouin P, Greenberg R, et al. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology. 1992;182:319–23. doi: 10.1148/radiology.182.2.1732943. [DOI] [PubMed] [Google Scholar]
- [17].Suzuki K, Nagai K, Yoshida J, Nishimura M, Takahashi K, Nishiwaki Y. Clinical predictors of N2 disease in the setting of a negative computed tomographic scan in patients with lung cancer. J Thorac Cardiovasc Surg. 1999;117:593–8. doi: 10.1016/s0022-5223(99)70340-5. [DOI] [PubMed] [Google Scholar]
- [18].Kernstine KH, Stanford W, Mullan BF, et al. PET, CT, and MRI with Combidex for mediastinal staging in non-small cell lung carcinoma. Ann Thorac Surg. 1999;68:1022–8. doi: 10.1016/s0003-4975(99)00788-2. [DOI] [PubMed] [Google Scholar]
- [19].Gupta NC, Graeber GM, Rogers JS, 2nd , Bishop HA. Comparative efficacy of positron emission tomography with FDG and computed tomographic scanning in preoperative staging of non-small cell lung cancer. Ann Surg. 1999;229:286–91. doi: 10.1097/00000658-199902000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Birim O, Kappetein AP, Stijnen T, Bogers AJ. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg. 2005;79:375–82. doi: 10.1016/j.athoracsur.2004.06.041. [DOI] [PubMed] [Google Scholar]
- [21].Dwamena B, Sonnad S, Angobaldo J, Wahl R. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s- meta-analytic comparison of PET and CT. Radiology. 1999;213:530–6. doi: 10.1148/radiology.213.2.r99nv46530. [DOI] [PubMed] [Google Scholar]
- [22].Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–19. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- [23].Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–7. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- [24].Cerfolio RJ, Ojha B, Bryant AS, Raghuveer V, Mountz JM, Bartolucci AA. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg. 2004;78:1017–1023. doi: 10.1016/j.athoracsur.2004.02.067. (discussion 1017–23) [DOI] [PubMed] [Google Scholar]
- [25].Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- [26].Chapet O, Kong FM, Quint LE, et al. CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys. 2005;63:170–8. doi: 10.1016/j.ijrobp.2004.12.060. [DOI] [PubMed] [Google Scholar]
- [27].Kelly RF, Tran T, Holmstrom A, Murar J, Segurola RJ., Jr Accuracy and cost-effectiveness of [18F]-2-fluoro-deoxy-D-glucose-positron emission tomography scan in potentially resectable non-small cell lung cancer. Chest. 2004;125:1413–23. doi: 10.1378/chest.125.4.1413. [DOI] [PubMed] [Google Scholar]
- [28].Weiss W, Gillick JS. The metastatic spread of bronchogenic carcinoma in relation to the interval between resection and death. Chest. 1977;71:725–9. doi: 10.1378/chest.71.6.725. [DOI] [PubMed] [Google Scholar]
- [29].Smith RA. Evaluation of long-term results of surgery for bronchial carcinoma. J Thorac Cardiovasc Surg. 1981;82:325–33. [PubMed] [Google Scholar]
- [30].Bell J. Abdominal exploration in one-hundred lung carcinoma suspects prior to thoracotomy. Ann Surg. 1968;167:199–203. doi: 10.1097/00000658-196802000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Matthews MJ, Kanhouwa S, Pickren J, Robinette D. Frequency of residual and metastatic tumor in patients undergoing curative surgical resection for lung cancer. Cancer Chemother Rep Part 3. 1973;4:63–7. [PubMed] [Google Scholar]
- [32].Quint L, Tummala S, Brisson L, et al. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thorac Surg. 1996;62:246. doi: 10.1016/0003-4975(96)00220-2. [DOI] [PubMed] [Google Scholar]
- [33].Korobkin M. CT characterization of adrenal masses: the time has come. Radiology. 2000;217:629–32. doi: 10.1148/radiology.217.3.r00dc52629. [DOI] [PubMed] [Google Scholar]
- [34].Caoili EM, Korobkin M, Francis IR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002;222:629–33. doi: 10.1148/radiol.2223010766. [DOI] [PubMed] [Google Scholar]
- [35].Blake MA, Kalra MK, Sweeney AT, et al. Distinguishing benign from malignant adrenal masses: multi-detector row CT protocol with 10-minute delay. Radiology. 2006;238:578–85. doi: 10.1148/radiol.2382041514. [DOI] [PubMed] [Google Scholar]
- [36].Jhaveri KS, Wong F, Ghai S, Haider MA. Comparison of CT histogram analysis and chemical shift MRI in the characterization of indeterminate adrenal nodules. Am J Roentgenol. 2006;187:1303–8. doi: 10.2214/AJR.05.1022. [DOI] [PubMed] [Google Scholar]
- [37].Savci G, Yazici Z, Sahin N, Akgoz S, Tuncel E. Value of chemical shift subtraction MRI in characterization of adrenal masses. Am J Roentgenol. 2006;186:130–5. doi: 10.2214/AJR.04.1370. [DOI] [PubMed] [Google Scholar]
- [38].Kumar R, Xiu Y, Yu JQ, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004;45:2058–62. [PubMed] [Google Scholar]
- [39].Erasmus JJ, Patz EF, Jr , McAdams HP, et al. Evaluation of adrenal masses in patients with bronchogenic carcinoma using 18F-fluorodeoxyglucose positron emission tomography. AJR. 1997;168:1357–60. doi: 10.2214/ajr.168.5.9129444. [DOI] [PubMed] [Google Scholar]
- [40].Caoili EM, Korobkin M, Brown RK, Mackie G, Shulkin BL. Differentiating adrenal adenomas from nonadenomas using (18)F-FDG PET/CT quantitative and qualitative evaluation. Acad Radiol. 2007;14:468–75. doi: 10.1016/j.acra.2007.01.009. [DOI] [PubMed] [Google Scholar]
- [41].Yun M, Kim W, Alnafisi N, Lacorte L, Jang S, Alavi A. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med. 2001;42:1795–9. [PubMed] [Google Scholar]
- [42].MacManus M, Hicks R, Matthews J, et al. High rate of detection of unsuspected distant metastases by PET in apparent Stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:287–93. doi: 10.1016/s0360-3016(01)01477-8. [DOI] [PubMed] [Google Scholar]
- [43].Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803–9. doi: 10.1148/radiology.212.3.r99se21803. [DOI] [PubMed] [Google Scholar]
- [44].Valk PE, Pounds TR, Hopkins DM, et al. Staging non-small cell lung cancer by whole-body positron emission tomographic imaging. Ann Thorac Surg. 1995;60:1573–81. doi: 10.1016/0003-4975(95)00752-0. (discussion 1581–1572_. [DOI] [PubMed] [Google Scholar]
- [45].Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–61. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- [46].Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- [47].Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- [48].Postmus PE, Brambilla E, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol. 2007;2:686–93. doi: 10.1097/JTO.0b013e31811f4703. [DOI] [PubMed] [Google Scholar]
- [49].Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–2. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- [50].Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:603–12. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- [51].Changlai SP, Tsai SC, Chou MC, Ho YJ, Kao CH. Whole body 18F-2-deoxyglucose positron emission tomography to restage non-small cell lung cancer. Oncol Rep. 2001;8:337–9. [PubMed] [Google Scholar]
- [52].Weng E, Tran L, Rege S, et al. Accuracy and clinical impact of mediastinal lymph node staging with FDG-PET imaging in potentially resectable lung cancer. Am J Clin Oncol. 2000;23:47–52. doi: 10.1097/00000421-200002000-00014. [DOI] [PubMed] [Google Scholar]
- [53].van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359:1388–93. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- [54].National Institute for Clinical Excellence. The diagnosis and treatment of lung cancer. 2005 http://guidance.nice.org.uk/ [Google Scholar]
- [55].Quint L. Lung cancer: CT staging. Cancer Imaging. 2004;4:S1–5. [Google Scholar]
- [56].Quint L, Francis I, Gross B. Conventional imaging of non-small cell lung cancer. In: Pass H, Carbone D, Johnson D, Minna J, Turrisi A, editors. Lung cancer: principles and practice. 3rd. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 315–44. [Google Scholar]