Abstract
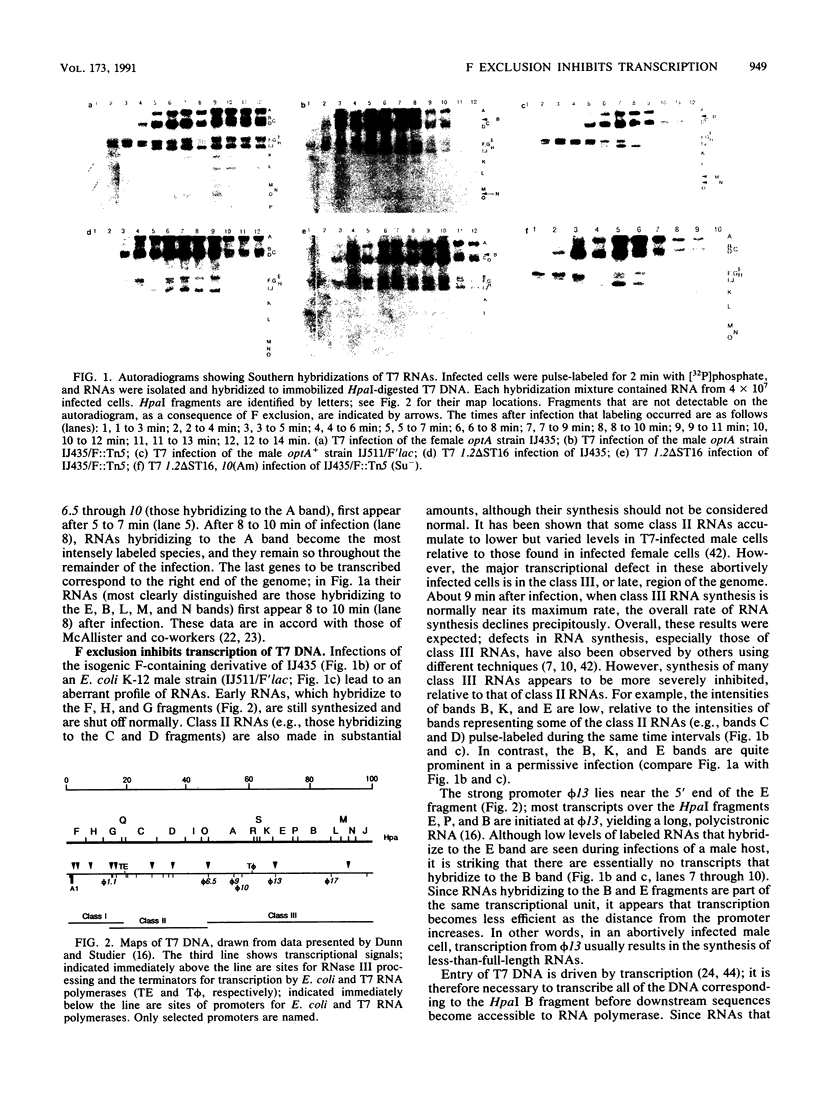
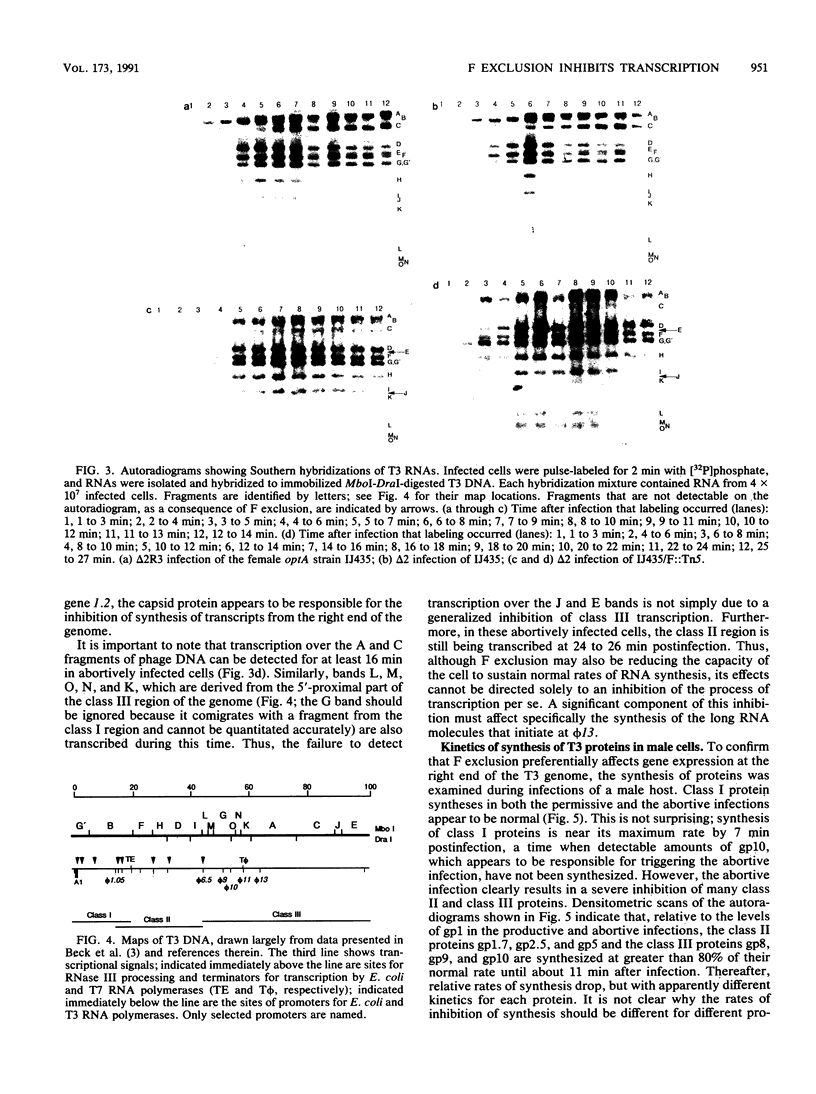
Transcription of T7 and mutant T3 DNA during infections of F plasmid-containing cells has been analyzed by using Southern hybridization. A transcriptional defect is apparent in these abortively infected cells that is most severe in the class III region of the phage genome. In particular, RNAs that are initiated from the gene 13 promoter are not elongated to give full-length molecules. It is suggested that the transcription defect results from positive supercoiling of the template DNA and that torsional constraints may even prevent the complete entry of the phage genome into an abortively infected cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird J. P., Bourguignon G. J., Sternglanz R. Effect of nalidixic acid on the growth of deoxyribonucleic acid bacteriophages. J Virol. 1972 Jan;9(1):17–21. doi: 10.1128/jvi.9.1.17-21.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp B. B., Richardson C. C. A unique deoxyguanosine triphosphatase is responsible for the optA1 phenotype of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2563–2567. doi: 10.1073/pnas.85.8.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P. J., Gonzalez S., Ward C. L., Molineux I. J. Sequence of bacteriophage T3 DNA from gene 2.5 through gene 9. J Mol Biol. 1989 Dec 20;210(4):687–701. doi: 10.1016/0022-2836(89)90102-2. [DOI] [PubMed] [Google Scholar]
- Beier H., Golomb M., Chamberlin M. Isolation of recombinants between T7 and T3 bacteriophages and their use in vitro transcriptional mapping. J Virol. 1977 Feb;21(2):753–765. doi: 10.1128/jvi.21.2.753-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D., Malamy M. H. Evidence for the presence of nontranslated T7 late mRNA in infected F'(PIF+) episome-containing cells. J Virol. 1974 Feb;13(2):378–385. doi: 10.1128/jvi.13.2.378-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Macromolecular synthesis in T7 infected F' cells. Virology. 1975 Sep;67(1):264–275. doi: 10.1016/0042-6822(75)90423-7. [DOI] [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Permeability lesions in male Escherichia coli infected with bacteriophage T7. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2222–2226. doi: 10.1073/pnas.72.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. A link between streptomycin and rifampicin mutation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2084–2087. doi: 10.1073/pnas.72.6.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Gorini L. Growth of bacteriophages MS2 and T7 on streptomycin-resistant mutants of Escherichia coli. J Bacteriol. 1975 Feb;121(2):670–674. doi: 10.1128/jb.121.2.670-674.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Steitz J. A. F factor-mediated inhibition of bacteriophage T7 growth: analysis of T7 RNA and protein synthesis in vivo and in vitro using male and female Escherichia coli. J Mol Biol. 1975 Oct 15;98(1):31–43. doi: 10.1016/s0022-2836(75)80099-4. [DOI] [PubMed] [Google Scholar]
- Condreay J. P., Molineux I. J. Synthesis of the capsid protein inhibits development of bacteriophage T3 mutants that abortively infect F plasmid-containing cells. J Mol Biol. 1989 Jun 5;207(3):543–554. doi: 10.1016/0022-2836(89)90463-4. [DOI] [PubMed] [Google Scholar]
- Condreay J. P., Wright S. E., Molineux I. J. Nucleotide sequence and complementation studies of the gene 10 region of bacteriophage T3. J Mol Biol. 1989 Jun 5;207(3):555–561. doi: 10.1016/0022-2836(89)90464-6. [DOI] [PubMed] [Google Scholar]
- De Wyngaert M., Hinkle D. C. Involvement of DNA gyrase in replication and transcription of bacteriophage T7 DNA. J Virol. 1979 Feb;29(2):529–535. doi: 10.1128/jvi.29.2.529-535.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H., Glenn J., McCorquodale D. J. Inhibition of bacteriophage replication by extrachromosomal genetic elements. Microbiol Rev. 1981 Mar;45(1):52–71. doi: 10.1128/mr.45.1.52-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Nucleotide sequence from the genetic left end of bacteriophage T7 DNA to the beginning of gene 4. J Mol Biol. 1981 Jun 5;148(4):303–330. doi: 10.1016/0022-2836(81)90178-9. [DOI] [PubMed] [Google Scholar]
- Huber H. E., Beauchamp B. B., Richardson C. C. Escherichia coli dGTP triphosphohydrolase is inhibited by gene 1.2 protein of bacteriophage T7. J Biol Chem. 1988 Sep 25;263(27):13549–13556. [PubMed] [Google Scholar]
- Kennedy M., Chandler M., Lane D. Mapping and regulation of the pifC promoter of the F plasmid. Biochim Biophys Acta. 1988 May 6;950(1):75–80. doi: 10.1016/0167-4781(88)90075-9. [DOI] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister W. T., Morris C., Rosenberg A. H., Studier F. W. Utilization of bacteriophage T7 late promoters in recombinant plasmids during infection. J Mol Biol. 1981 Dec 15;153(3):527–544. doi: 10.1016/0022-2836(81)90406-x. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Wu H. L. Regulation of transcription of the late genes of bacteriophage T7. Proc Natl Acad Sci U S A. 1978 Feb;75(2):804–808. doi: 10.1073/pnas.75.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt B. A., Studier F. W. Entry of bacteriophage T7 DNA into the cell and escape from host restriction. J Bacteriol. 1988 May;170(5):2095–2105. doi: 10.1128/jb.170.5.2095-2105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineux I. J., Schmitt C. K., Condreay J. P. Mutants of bacteriophage T7 that escape F restriction. J Mol Biol. 1989 Jun 5;207(3):563–574. doi: 10.1016/0022-2836(89)90465-8. [DOI] [PubMed] [Google Scholar]
- Molineux I. J., Spence J. L. Virus-plasmid interactions: mutants of bacteriophage T3 that abortively infect plasmid F-containing (F+) strains of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1465–1469. doi: 10.1073/pnas.81.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Blumberg D. D., Malamy M. H. T7 protein synthesis in F' episome-containing cells: assignment of specific proteins to three translational groups. J Virol. 1974 Feb;13(2):386–393. doi: 10.1128/jvi.13.2.386-393.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Myers J. A., Beauchamp B. B., Richardson C. C. Gene 1.2 protein of bacteriophage T7. Effect on deoxyribonucleotide pools. J Biol Chem. 1987 Apr 15;262(11):5288–5292. [PubMed] [Google Scholar]
- Remes B., Elseviers D. Adenosine 5'-triphosphate leakage does not cause abortive infection of bacteriophage T7 in male Escherichia coli. J Bacteriol. 1980 Aug;143(2):1054–1056. doi: 10.1128/jb.143.2.1054-1056.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Genetic analysis of gene 1.2 of bacteriophage T7: isolation of a mutant of Escherichia coli unable to support the growth of T7 gene 1.2 mutants. J Virol. 1981 Jan;37(1):343–351. doi: 10.1128/jvi.37.1.343-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Beck P. J., Kearney C. A., Spence J. L., DiGiovanni D., Condreay J. P., Molineux I. J. Sequence of a conditionally essential region of bacteriophage T3, including the primary origin of DNA replication. J Mol Biol. 1987 Feb 5;193(3):479–495. doi: 10.1016/0022-2836(87)90261-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steck T. R., Drlica K. Involvement of DNA gyrase in bacteriophage T7 growth. J Virol. 1985 Jan;53(1):296–298. doi: 10.1128/jvi.53.1.296-298.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic mapping of a mutation that causes ribonucleases III deficiency in Escherichia coli. J Bacteriol. 1975 Oct;124(1):307–316. doi: 10.1128/jb.124.1.307-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Relationships among different strains of T7 and among T7-related bacteriophages. Virology. 1979 May;95(1):70–84. doi: 10.1016/0042-6822(79)90402-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Yamada M., Fujisawa H., Kato H., Hamada K., Minagawa T. Cloning and sequencing of the genetic right end of bacteriophage T3 DNA. Virology. 1986 Jun;151(2):350–361. doi: 10.1016/0042-6822(86)90055-3. [DOI] [PubMed] [Google Scholar]
- Young E. T., Menard R. C. Analysis of the template activity of bacteriophage T7 messenger RNAs during infection of male and female strains of Escherichia coli. J Mol Biol. 1975 Nov 25;99(1):167–184. doi: 10.1016/s0022-2836(75)80166-5. [DOI] [PubMed] [Google Scholar]
- Zavriev S. K., Shemyakin M. F. RNA polymerase-dependent mechanism for the stepwise T7 phage DNA transport from the virion into E. coli. Nucleic Acids Res. 1982 Mar 11;10(5):1635–1652. doi: 10.1093/nar/10.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]