Abstract
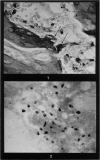
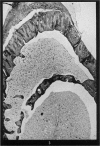
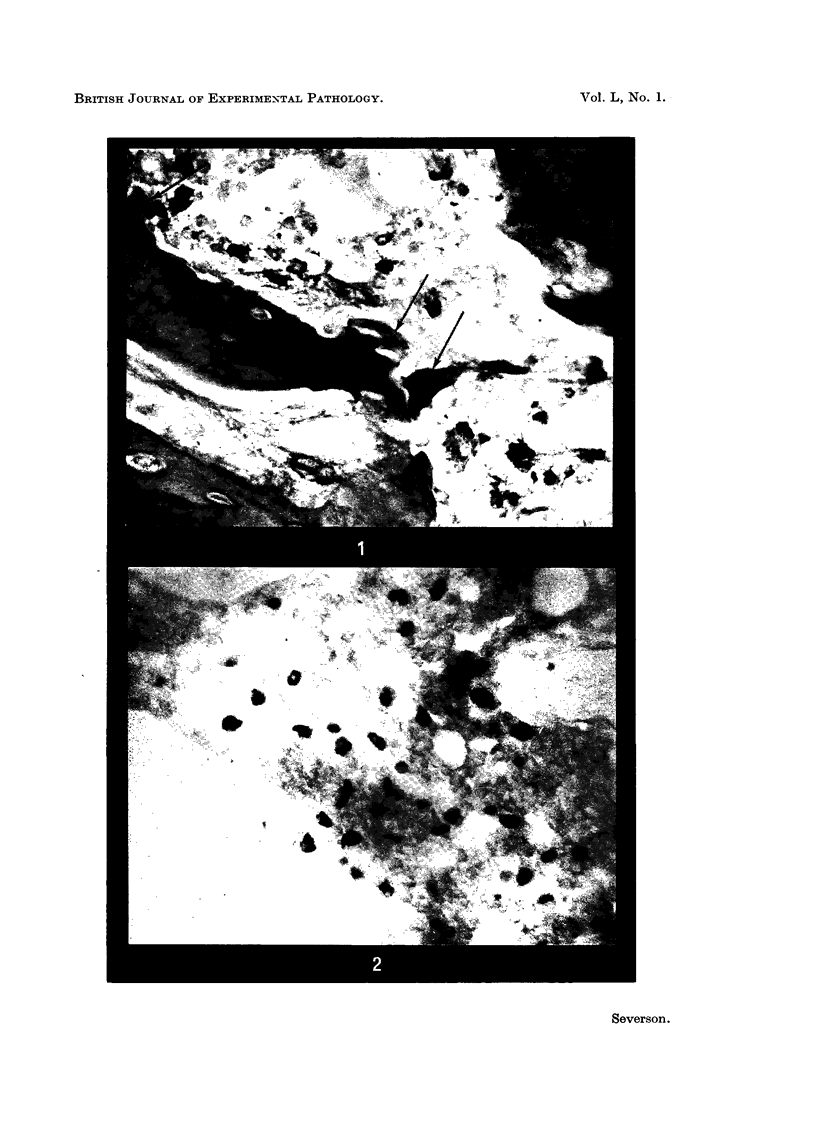
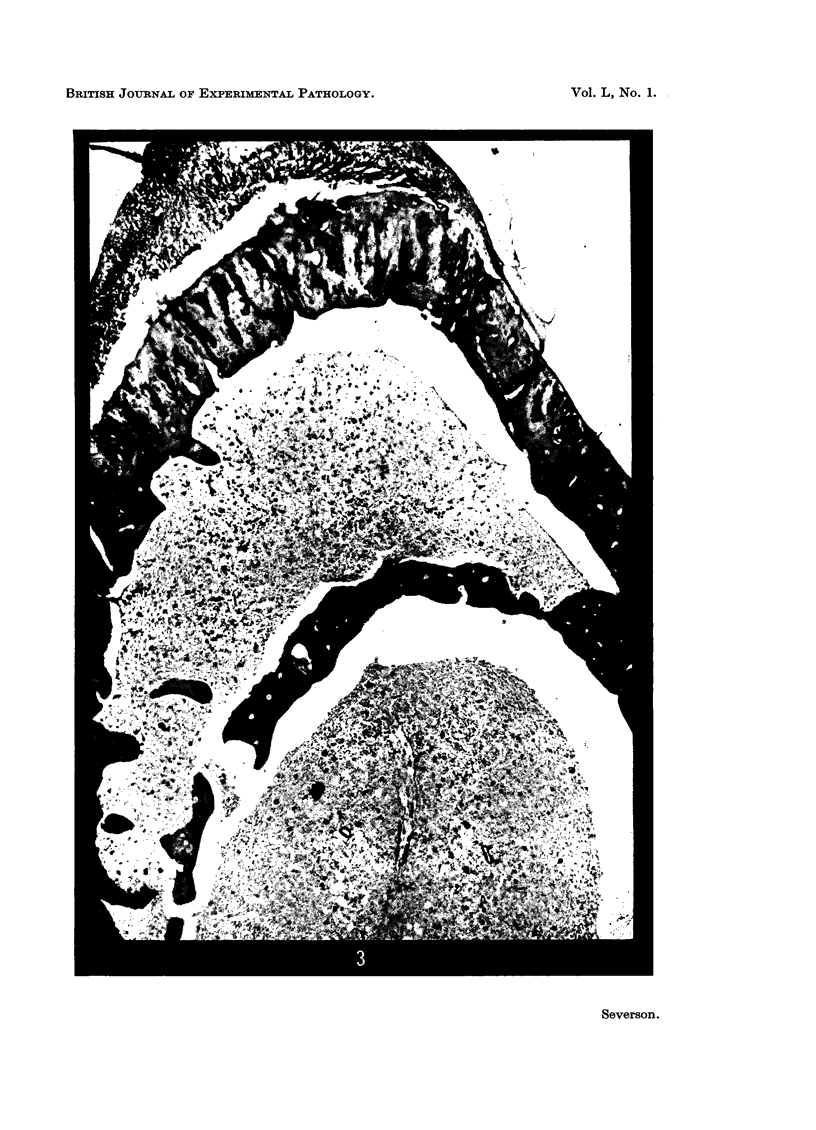
Investigation on the presence of mast cells in the remodelling adductor longus-pectineus exostosis, induced by either surgical or BAPN stimulation, and in the healing fracture callus, demonstrated that mast cells were not pathognomonic for the bone lesions of BAPN-treated animals. Numerous mast cells were found in association with the endosteum of the skeletal trabeculae and throughout the new marrow replacing the trabeculae of the experimental lesions, with no increase in the mast cell numbers in the original marrow of the femoral shaft. Mast cells were not present in areas of cartilage resorption, but they were present when resorption and remodelling of the endochondral trabeculae commenced. It is surmised that mast cells play a role in connective tissue resorption and remodelling by supplying a factor necessary for the release of hydrolytic enzymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GOLDHABER P. HEPARIN ENHANCEMENT OF FACTORS STIMULATING BONE RESORPTION IN TISSUE CULTURE. Science. 1965 Jan 22;147(3656):407–408. doi: 10.1126/science.147.3656.407. [DOI] [PubMed] [Google Scholar]
- JAFFE M. D., WILLIS P. W., 3rd MULTIPLE FRACTURES ASSOCIATED WITH LONG-TERM SODIUM HEPARIN THERAPY. JAMA. 1965 Jul 12;193:158–160. doi: 10.1001/jama.1965.03090020072024. [DOI] [PubMed] [Google Scholar]
- Kaufman E. J., Glimcher M. J., Mechanic G. L., Goldhaber P. Collagenolytic activity during active bone resorption in tissue culture. Proc Soc Exp Biol Med. 1965 Dec;120(3):632–637. doi: 10.3181/00379727-120-30611. [DOI] [PubMed] [Google Scholar]
- Lalich J. J., Groupner K., Jolin J. The influence of copper and molybdate salts on the production of bony deformities in rats. Lab Invest. 1965 Aug;14(8):1482–1493. [PubMed] [Google Scholar]
- Levy S. W. Effects of heparin in vivo on lysosomal enzymes in rat plasma. Can J Biochem. 1967 Jul;45(7):1145–1151. doi: 10.1139/o67-131. [DOI] [PubMed] [Google Scholar]
- Sognnaes R. F. Fluoride protection of bones and teeth. Science. 1965 Nov 19;150(3699):989–993. doi: 10.1126/science.150.3699.989. [DOI] [PubMed] [Google Scholar]
- TAKEOKA O., ANGEVINE D. M., LALICH J. J. PROLIFERATION OF MAST CELLS IN THE BONE MARROW OF RATS AFTER FEEDING BETA-AMINOPROPIONITRILE AND BETA-MERCAPTOETHYLAMINE. Am J Pathol. 1963 Oct;43:639–650. [PMC free article] [PubMed] [Google Scholar]
- URIST M. R., MCLEAN F. C. Accumulation of mast cells in endosteum of bones of calcium-deficient rats. AMA Arch Pathol. 1957 Mar;63(3):239–251. [PubMed] [Google Scholar]
- WISLOCKI G. B., SOGNNAES R. F. Histochemical reactions of normal teeth. Am J Anat. 1950 Sep;87(2):239–275. doi: 10.1002/aja.1000870204. [DOI] [PubMed] [Google Scholar]
- Yeager V. L., Taylor J. J. Periosteal response in lathyrism after semistarvation. Arch Pathol. 1965 Dec;80(6):647–650. [PubMed] [Google Scholar]