Abstract
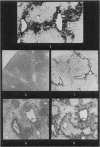
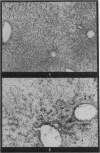
Autoradiography of mouse liver after the administration of 35S-sodium sulphate shows transient concentration of the isotope in sinusoidal walls. This phenomenon is markedly exaggerated in the acute stages of carbon tetrachloride (CCl4) poisoning with radioactivity appearing over viable sinusoidal cells and extracellular necrotic spaces. This is maintained for several days and there is condensation of the isotope over prominent reticulin fibres in the centrilobular zones during the stage of recovery. Negative results are obtained if the sinusoidal cells are included in the necrotic process as induced by CCl4 or by the local application of cold to the surface of the liver. It is concluded that liver sinusoidal cells have a capacity for mucopolysaccharide synthesis which is exaggerated following the induction of hepatocyte injury. There is also evidence for continuing synthesis of sulphated mucopolysaccharide in the cirrhotic liver in relation to fibrous trabeculae and hepatocyte degeneration.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREWS J. R., SWARM R. L., SCHLACHTER L., BRACE K. C., RUBIN P., BERGENSTAL D. M., GUMP H., SIEGEL S., SWAIN R. W. The effects of one curie of sulfur 35 administered intravenously as sulfate to a man with advanced chondrosarcoma. Am J Roentgenol Radium Ther Nucl Med. 1960 Jan;83:123–134. [PubMed] [Google Scholar]
- BERENSON G. S., DALFERES E. R. Identification of acid mucopolysaccharides from granulation tissue in rats. Br J Exp Pathol. 1960 Aug;41:422–429. [PMC free article] [PubMed] [Google Scholar]
- Bronfenmajer S., Schaffner F., Popper H. Fat-storing cells (lipocytes) in human liver. Arch Pathol. 1966 Nov;82(5):447–453. [PubMed] [Google Scholar]
- Burkel W. E., Low F. N. The fine structure of rat liver sinusoids, space of Dissé and associated tissue space. Am J Anat. 1966 May;118(3):769–783. doi: 10.1002/aja.1001180307. [DOI] [PubMed] [Google Scholar]
- Carr I., Kugler J. H. The failure of vascular endothelium to bind labelled sulphate. J Pathol Bacteriol. 1968 Jan;95(1):185–189. doi: 10.1002/path.1700950121. [DOI] [PubMed] [Google Scholar]
- Chvapil M. Conflicting hypotheses on experimental silicotic fibrogenesis: new experimental data. Environ Res. 1967 Jun;1(1):89–101. doi: 10.1016/0013-9351(67)90006-0. [DOI] [PubMed] [Google Scholar]
- Galambos J. T. Acid mucopolysaccharides and cirrhosis of the liver. Gastroenterology. 1966 Jul;51(1):65–74. [PubMed] [Google Scholar]
- ITO T., NEMOTO M. Uber die Kupfferschen Sternzellen und die Fettspeicherungszellen (fat storing cells) in der Blutkapillarenwand der memschlichen Leber. Okajimas Folia Anat Jpn. 1952 Oct;24(4):243–258. doi: 10.2535/ofaj1936.24.4_243. [DOI] [PubMed] [Google Scholar]
- JOHN D. W., MILLER L. L. INORGANIC S35 O-4= METABOLISM BY THE ISOLATED PERFUSED RAT LIVER. STUDIES ON THE NATURE OF S35 LABELING OF PLASMA PROTEINS. Lab Invest. 1965 Jul;14:1402–1411. [PubMed] [Google Scholar]
- MANCINI R. E., VILAR O., STEIN E., FIORINI H. A histochemical and radioautographic study of the participation of fibroblasts in the production of mucopolysaccharides in connective tissue. J Histochem Cytochem. 1961 May;9:278–291. doi: 10.1177/9.3.278. [DOI] [PubMed] [Google Scholar]
- MOWRY R. W. Improved procedure for the staining of acidic polysaccharides by Müller's colloidal (hydrous) ferric oxide and its combination with the Feulgen and the periodic acid-Schiff reactions. Lab Invest. 1958 Nov-Dec;7(6):566–576. [PubMed] [Google Scholar]
- Mathews M. B. The interaction of collagen and acid mucopolysaccharides. A model for connective tissue. Biochem J. 1965 Sep;96(3):710–716. doi: 10.1042/bj0960710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee J. O., Patrick R. S. The synthesis of sulphated mucopolysaccharide in mouse liver following carbon tetrachloride injury. II. Quantitation and partial characterization of extracted mucopolysaccharide. Br J Exp Pathol. 1969 Dec;50(6):527–532. [PMC free article] [PubMed] [Google Scholar]
- PATRICK R. S., KENNEDY J. S. THE SYNTHESIS OF SULPHATED MUCOPOLYSACCHARIDE AT SITES OF HEPATIC FIBROSIS INDUCED BY CARBON TETRACHLORIDE, AMYLOIDOSIS AND THE IMPLANTATION OF CATGUT. J Pathol Bacteriol. 1964 Oct;88:549–555. doi: 10.1002/path.1700880219. [DOI] [PubMed] [Google Scholar]
- Patek P. R., De Mignard A., Bernick S. Age changes in structure and responses of reticuloendothelial cells of rat liver. J Reticuloendothel Soc. 1967 May;4(2):211–218. [PubMed] [Google Scholar]
- Patrick R. S., McGee J. O. The utilisation of proline by the sinusoidal cells of mouse liver damaged by hepatotoxic agents. J Pathol Bacteriol. 1967 Jan;93(1):309–315. doi: 10.1002/path.1700930129. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr, Ashley C. A. An artefact in radioautography due to binding of free amino acids to tissues by fixatives. J Cell Biol. 1967 Apr;33(1):53–60. doi: 10.1083/jcb.33.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E. Autoradiographic characterization of sulfated acid mucopolysaccharides in experimental cirrhosis. J Histochem Cytochem. 1966 Sep;14(9):688–689. doi: 10.1177/14.9.688. [DOI] [PubMed] [Google Scholar]
- Rubin E., Hutterer F., Popper H. Experimental hepatic fibrosis without hepatocellular regeneration. A kinetic study. Am J Pathol. 1968 Jan;52(1):111–120. [PMC free article] [PubMed] [Google Scholar]
- SCHAFFNER F., BARKA T., POPPER H. HEPATIC MESENCHYMAL CELL REACTION IN LIVER DISEASE. Exp Mol Pathol. 1963 Oct;31:419–441. doi: 10.1016/0014-4800(63)90020-0. [DOI] [PubMed] [Google Scholar]
- TIGHE J. R., MEACHIM G. Sulphate utilisation by mucinous and other connective-tissue tumours. J Pathol Bacteriol. 1962 Jan;83:195–205. doi: 10.1002/path.1700830122. [DOI] [PubMed] [Google Scholar]
- Takada A., Porta E. A., Hartroft W. S. The recovery of experimental dietary cirrhosis. II. Turnover changes of hepatic cells and collagen. Am J Pathol. 1967 Dec;51(6):959–976. [PMC free article] [PubMed] [Google Scholar]
- WOOD G. C. The formation of fibrils from collagen solutions. 3. Effect of chondroitin sulphate and some other naturally occurring polyanions on the rate of formation. Biochem J. 1960 Jun;75:605–612. doi: 10.1042/bj0750605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD R. L. Evidence of species differences in the ultrastructure of the hepatic sinusoid. Z Zellforsch Mikrosk Anat. 1963;58:679–692. doi: 10.1007/BF00410656. [DOI] [PubMed] [Google Scholar]