Abstract
The velocity, run time, path curvature, and reorientation angle of Chromatium minus were measured as a function of light intensity, temperature, viscosity, osmotic pressure, and hydrogen sulfide concentration. C. minus changed both velocity and run time. Velocity decreased with increasing light intensity in sulfide-depleted cultures and increased in sulfide-replete cultures. The addition of sulfide to cultures grown at low light intensity (10 microeinsteins m-2 s-1) caused mean run times to increase from 10.5 to 20.6 s. The addition of sulfide to cultures grown at high light intensity (100 microeinsteins m-2 s-1) caused mean run times to decrease from 15.3 to 7.7 s. These changes were maintained for up to an hour and indicate that at least some members of the family Chromatiaceae simultaneously modulate velocity and turning frequency for extended periods as part of normal taxis.
Full text
PDF

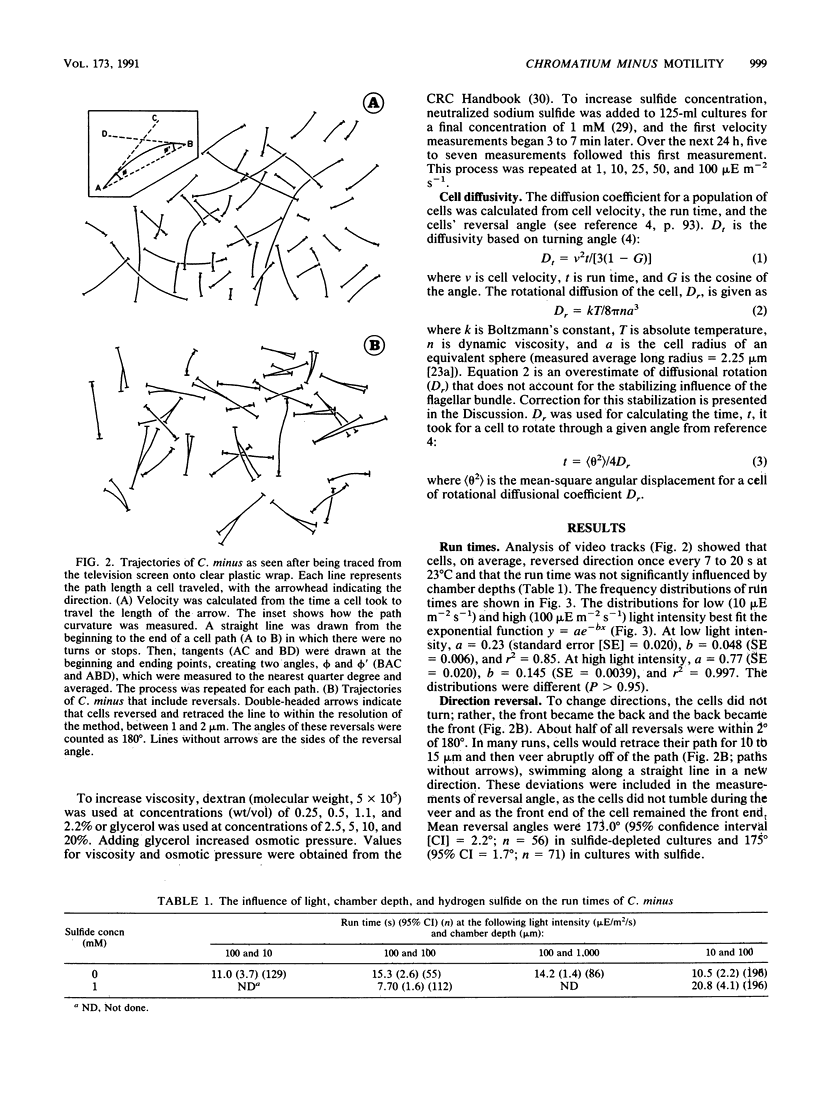
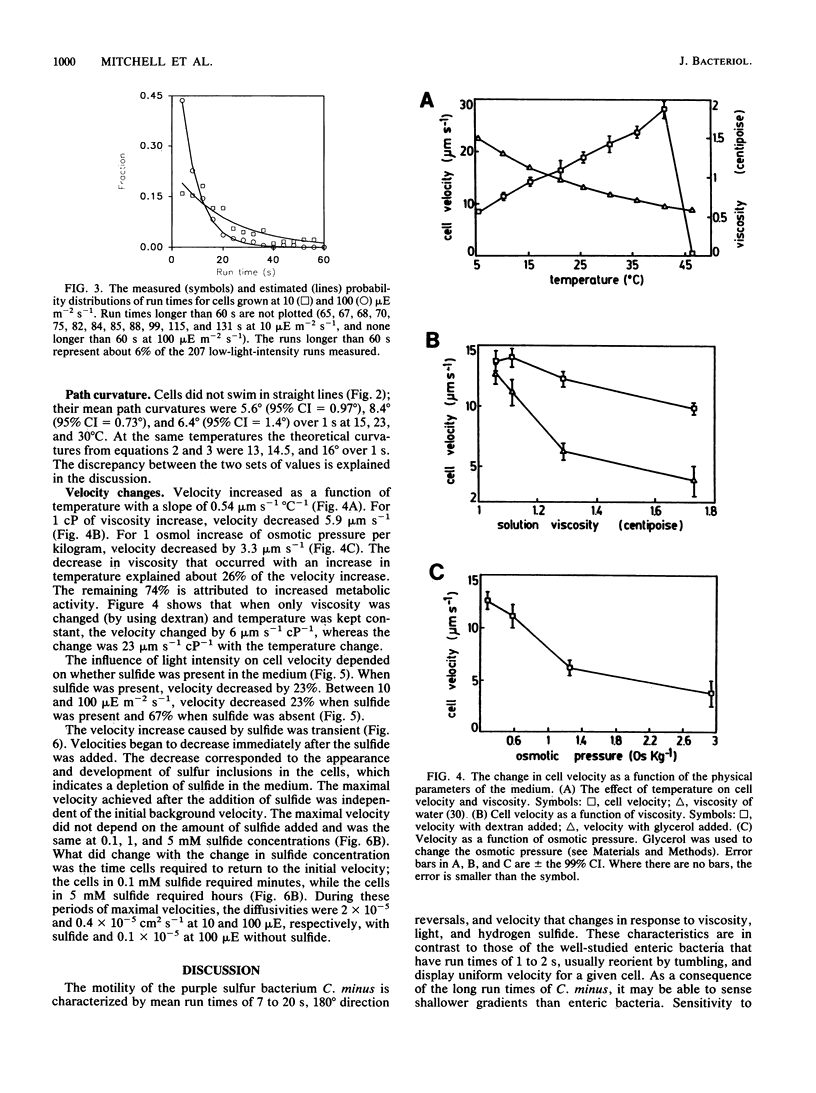
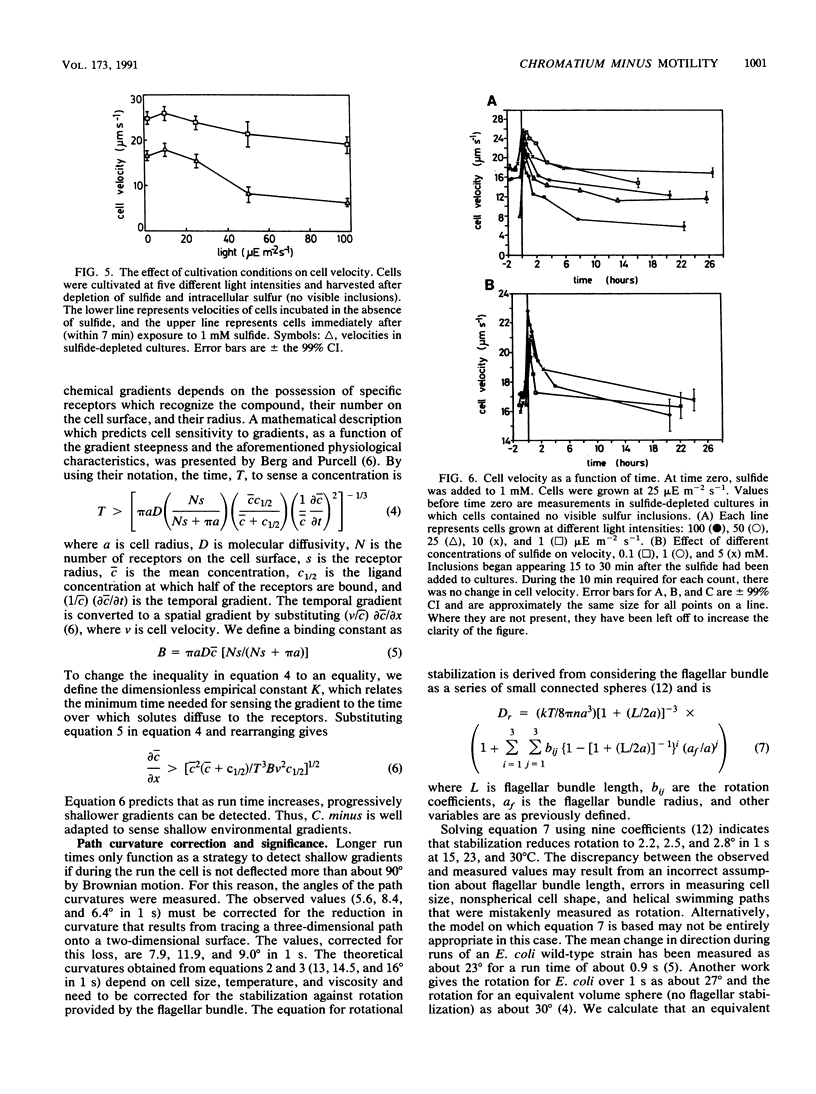


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage J. P., Macnab R. M. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides. J Bacteriol. 1987 Feb;169(2):514–518. doi: 10.1128/jb.169.2.514-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Turner L. Movement of microorganisms in viscous environments. Nature. 1979 Mar 22;278(5702):349–351. doi: 10.1038/278349a0. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. On the interplay of environmental factors affecting taxis and motility in Rhodospirillum rubrum. Arch Mikrobiol. 1958;29(2):189–212. doi: 10.1007/BF00409860. [DOI] [PubMed] [Google Scholar]
- Fosnaugh K., Greenberg E. P. Motility and chemotaxis of Spirochaeta aurantia: computer-assisted motion analysis. J Bacteriol. 1988 Apr;170(4):1768–1774. doi: 10.1128/jb.170.4.1768-1774.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de la Torre J. G., Bloomfield V. A. Hydrodynamic properties of complex, rigid, biological macromolecules: theory and applications. Q Rev Biophys. 1981 Feb;14(1):81–139. doi: 10.1017/s0033583500002080. [DOI] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Motility of flagellated bacteria in viscous environments. J Bacteriol. 1977 Oct;132(1):356–358. doi: 10.1128/jb.132.1.356-358.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Park C., Nowlin D. M. Adaptational "crosstalk" and the crucial role of methylation in chemotactic migration by Escherichia coli. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1448–1452. doi: 10.1073/pnas.86.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B., DiSpirito A. A. In bacteria which grow on simple reductants, generation of a proton gradient involves extracytoplasmic oxidation of substrate. Microbiol Rev. 1985 Jun;49(2):140–157. doi: 10.1128/mr.49.2.140-157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr A response regulator model in a simple sensory system. Science. 1977 Jun 3;196(4294):1055–1063. doi: 10.1126/science.870969. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Bacterial flagella rotating in bundles: a study in helical geometry. Proc Natl Acad Sci U S A. 1977 Jan;74(1):221–225. doi: 10.1073/pnas.74.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Imae Y., Shioi J. I., Oosawa F. Effect of temperature on motility and chemotaxis of Escherichia coli. J Bacteriol. 1976 Sep;127(3):1039–1046. doi: 10.1128/jb.127.3.1039-1046.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos E. Change in Size of Chromatium minus Cells in Relation to Growth Rate, Sulfur Content, and Photosynthetic Activity: A Comparison of Pure Cultures and Field Populations. Appl Environ Microbiol. 1987 Apr;53(4):864–871. doi: 10.1128/aem.53.4.864-871.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFENNIG N. [Observations on swarming of Chromatium okenii]. Arch Mikrobiol. 1962;42:90–95. [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]