Abstract
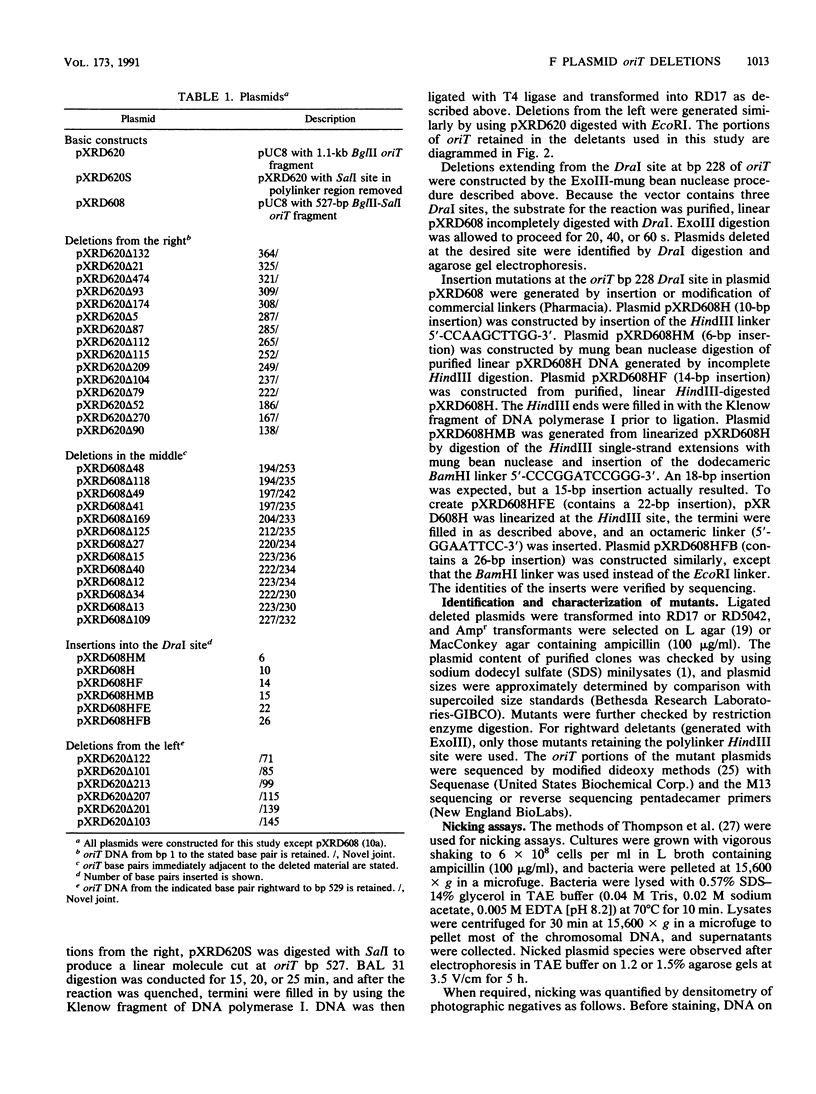
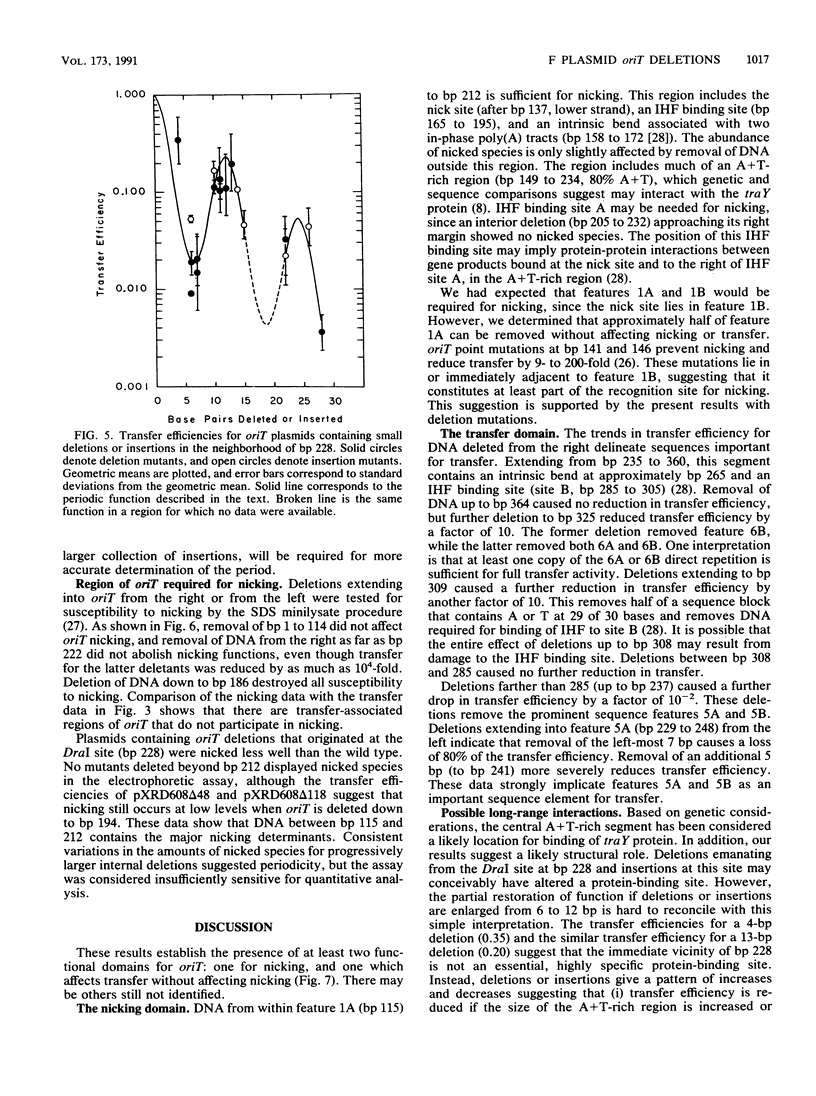
Functional domains of the Escherichia coli F plasmid oriT locus were identified by deletion analysis. DNA sequences required for nicking or transfer were revealed by cloning deleted segments of oriT into otherwise nonmobilizable pUC8 vectors and testing for their ability to promote transfer or to be nicked when tra operon functions were provided in trans. Removal of DNA sequences to the right of the central A + T-rich region (i.e., from the direction of traM) did not affect the susceptibility of oriT to nicking functions; however, transfer efficiency for oriT segments deleted from the right was progressively reduced over an 80- to 100-bp interval. Deletions extending toward the oriT nick site from the left did not affect the frequency of transfer if deletion endpoints lay at least 22 bp away from the nick site. Deletions or insertions in the central, A + T-rich region caused periodic variation in transfer efficiency, indicating that phase relationships between nicking and transfer domains of oriT must be preserved for full oriT function. These data show that the F oriT locus is extensive, with domains that individually contribute to transfer, nicking, and overall structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Deonier R. C., Mirels L. Excision of F plasmid sequences by recombination at directly repeated insertion sequence 2 elements: involvement of recA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3965–3969. doi: 10.1073/pnas.74.9.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S K, Adelberg E A. The Occurrence of a Genetic Transposition in a Strain of Escherichia Coli. Genetics. 1962 May;47(5):577–585. doi: 10.1093/genetics/47.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Horiuchi K., Zinder N. D. The functional origin of bacteriophage f1 DNA replication. Its signals and domains. J Mol Biol. 1984 Feb 5;172(4):507–521. doi: 10.1016/s0022-2836(84)80020-0. [DOI] [PubMed] [Google Scholar]
- Everett R., Willetts N. Characterisation of an in vivo system for nicking at the origin of conjugal DNA transfer of the sex factor F. J Mol Biol. 1980 Jan 15;136(2):129–150. doi: 10.1016/0022-2836(80)90309-5. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Origin of transfer of IncF plasmids and nucleotide sequences of the type II oriT, traM, and traY alleles from ColB4-K98 and the type IV traY allele from R100-1. J Bacteriol. 1986 Oct;168(1):132–139. doi: 10.1128/jb.168.1.132-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Ziegelin G., Kröger M., Lanka E. Conjugative transfer of promiscuous IncP plasmids: interaction of plasmid-encoded products with the transfer origin. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1771–1775. doi: 10.1073/pnas.86.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K. A., Minkley E. G., Jr The conjugation system of F, the fertility factor of Escherichia coli. Annu Rev Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- JACOB F., ADELBERG E. A. Transfert de caractéres génétiques par incorporation au facteur sexuel d'Escherichia coli. C R Hebd Seances Acad Sci. 1959 Jul 6;249(1):189–191. [PubMed] [Google Scholar]
- Johnston S., Ray D. S. Interference between M13 and oriM13 plasmids is mediated by a replication enhancer sequence near the viral strand origin. J Mol Biol. 1984 Aug 25;177(4):685–700. doi: 10.1016/0022-2836(84)90044-5. [DOI] [PubMed] [Google Scholar]
- Kim K., Meyer R. J. Unidirectional transfer of broad host-range plasmid R1162 during conjugative mobilization. Evidence for genetically distinct events at oriT. J Mol Biol. 1989 Aug 5;208(3):501–505. doi: 10.1016/0022-2836(89)90513-5. [DOI] [PubMed] [Google Scholar]
- Komano T., Toyoshima A., Morita K., Nisioka T. Cloning and nucleotide sequence of the oriT region of the IncI1 plasmid R64. J Bacteriol. 1988 Sep;170(9):4385–4387. doi: 10.1128/jb.170.9.4385-4387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lahue E. E., Matson S. W. Purified Escherichia coli F-factor TraY protein binds oriT. J Bacteriol. 1990 Mar;172(3):1385–1391. doi: 10.1128/jb.172.3.1385-1391.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Robberson D. L. Analysis and optimization of recombinant DNA joining reactions. J Mol Biol. 1985 Jan 20;181(2):297–312. doi: 10.1016/0022-2836(85)90093-2. [DOI] [PubMed] [Google Scholar]
- McIntire S. A., Dempsey W. B. oriT sequence of the antibiotic resistance plasmid R100. J Bacteriol. 1987 Aug;169(8):3829–3832. doi: 10.1128/jb.169.8.3829-3832.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Shure M., Vinograd J. The quantitation of fluorescence by photography. Nucleic Acids Res. 1977;4(5):1409–1418. doi: 10.1093/nar/4.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Taylor L., Kelly K., Everett R., Willetts N. The F plasmid origin of transfer: DNA sequence of wild-type and mutant origins and location of origin-specific nicks. EMBO J. 1984 May;3(5):1175–1180. doi: 10.1002/j.1460-2075.1984.tb01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. L., Centola M. B., Deonier R. C. Location of the nick at oriT of the F plasmid. J Mol Biol. 1989 Jun 5;207(3):505–512. doi: 10.1016/0022-2836(89)90460-9. [DOI] [PubMed] [Google Scholar]
- Tsai M. M., Fu Y. H., Deonier R. C. Intrinsic bends and integration host factor binding at F plasmid oriT. J Bacteriol. 1990 Aug;172(8):4603–4609. doi: 10.1128/jb.172.8.4603-4609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Location of the origin of transfer of the sex factor F. J Bacteriol. 1972 Nov;112(2):773–778. doi: 10.1128/jb.112.2.773-778.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]