Abstract
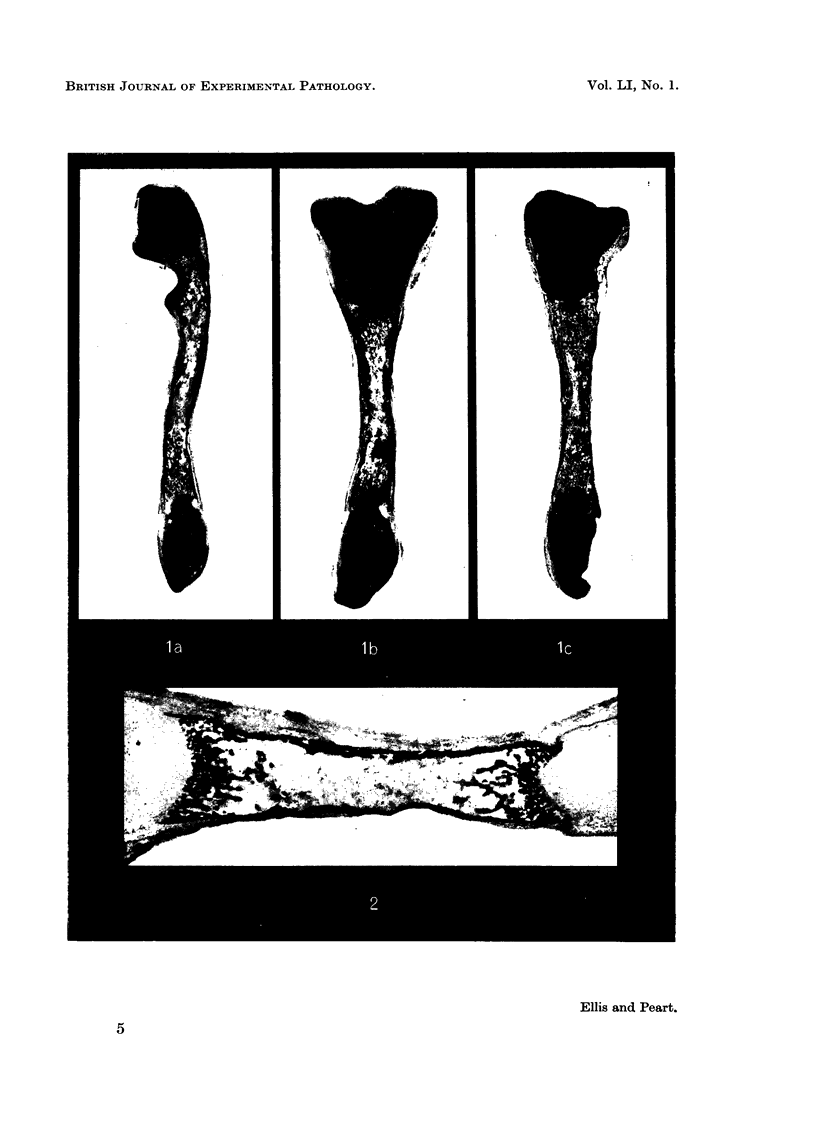
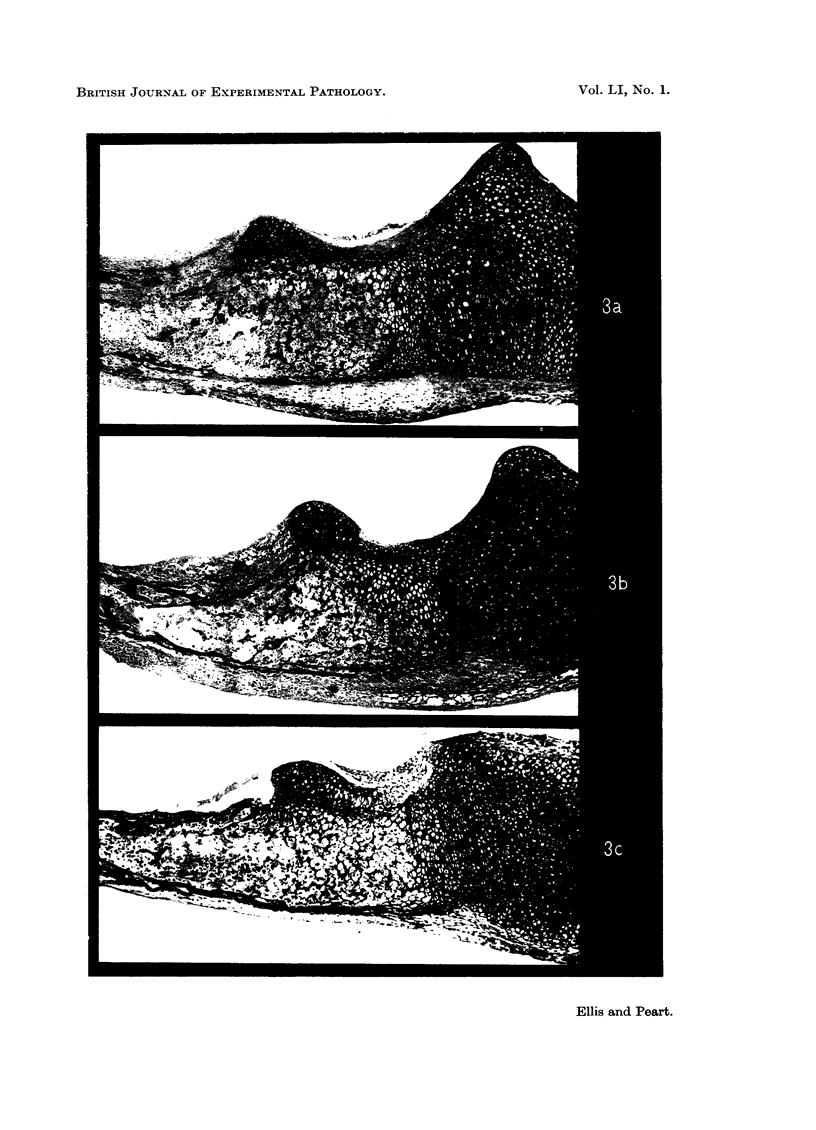
Heparin and the synthetic substitute dextran sulphate induce osteoporisis following prolonged administration to man or experimental animals. The possibility that this is brought about by a direct toxic effect on bone has been studied in tissue culture using explants of mouse radii, ulnae and tibiae. Fifty-three bones from new born or day old mice were cultured as controls, 73 with added heparin and 74 with added dextran sulphate at concentrations 0·1, 1·0 and 5·0 mg. per ml. Cultures were continued for 6 days. Control bones increased in length by approximately 22 per cent during this period and although little endochondral ossification occurred there was considerable periosteal and endosteal new bone formation.
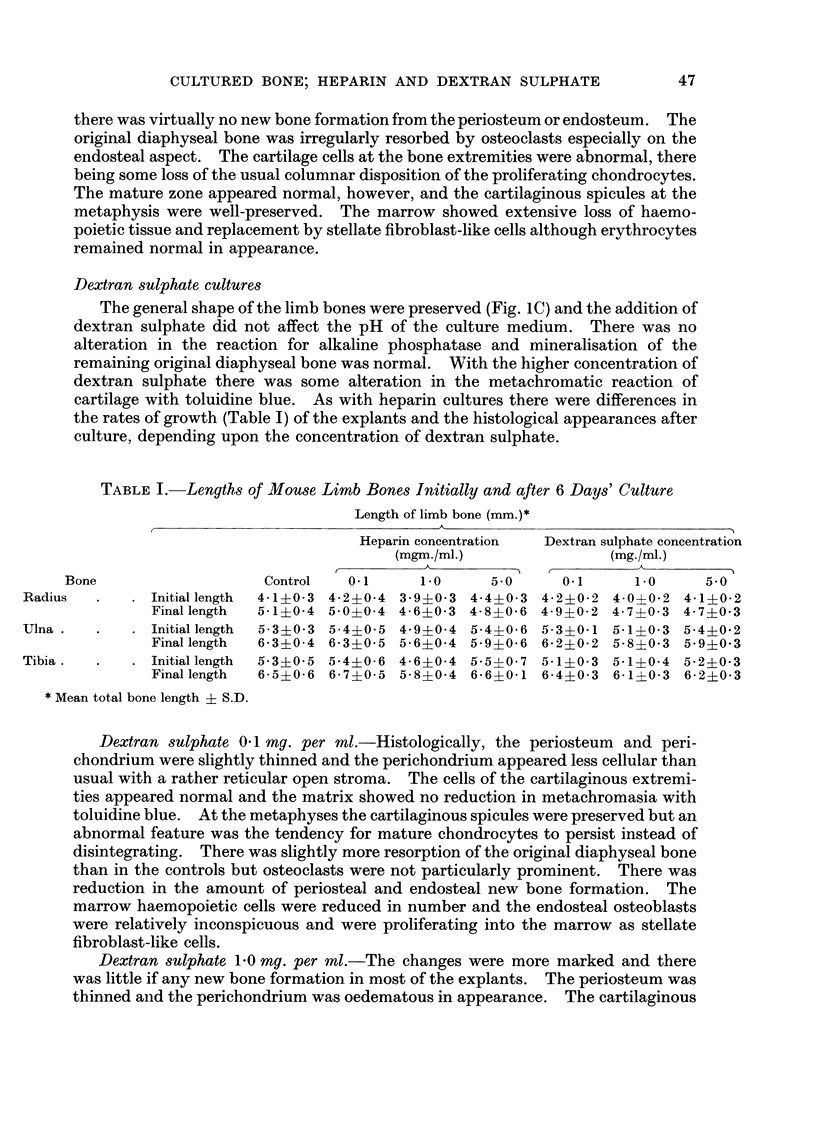
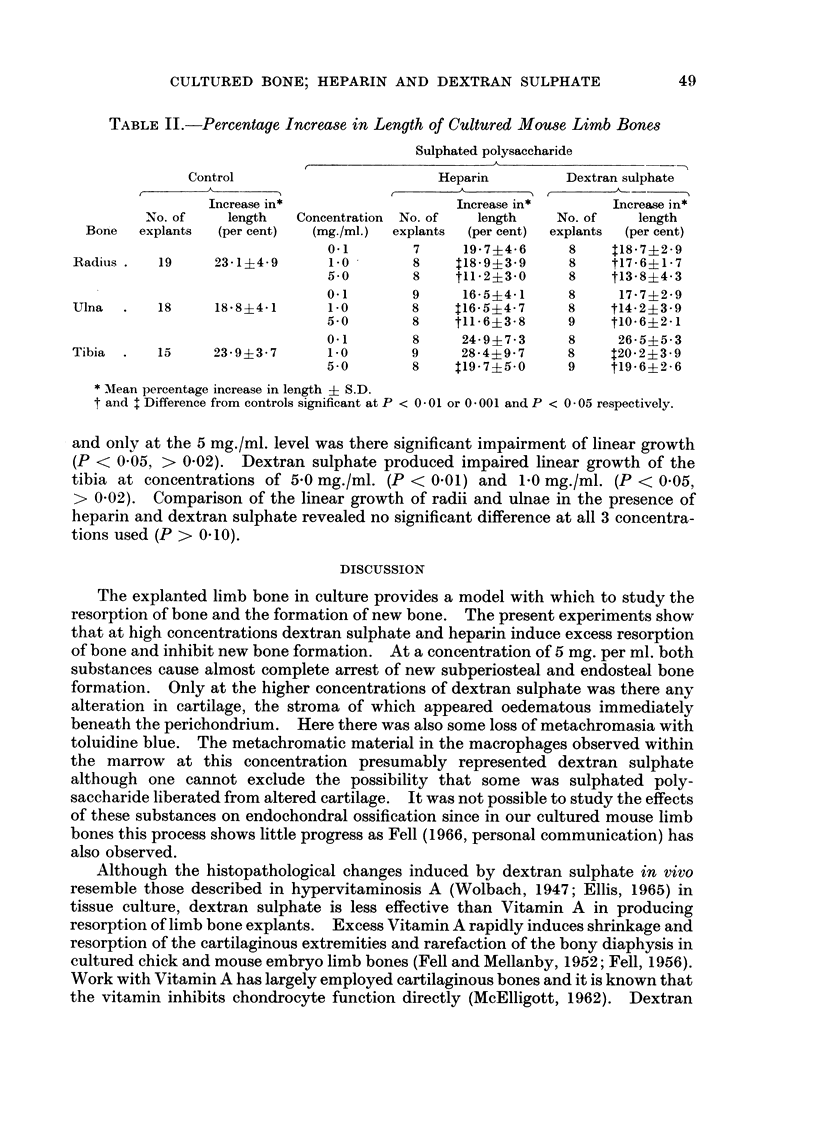
When heparin or dextran sulphate was added to the culture medium there was progressive impairment of linear growth with increasing concentrations of these substances. Thus at a concentration of 0·1 mg. per ml. there was little impairment of linear growth but at 5·0 mg. per ml. the bones increased in length by only approximately 15 per cent.
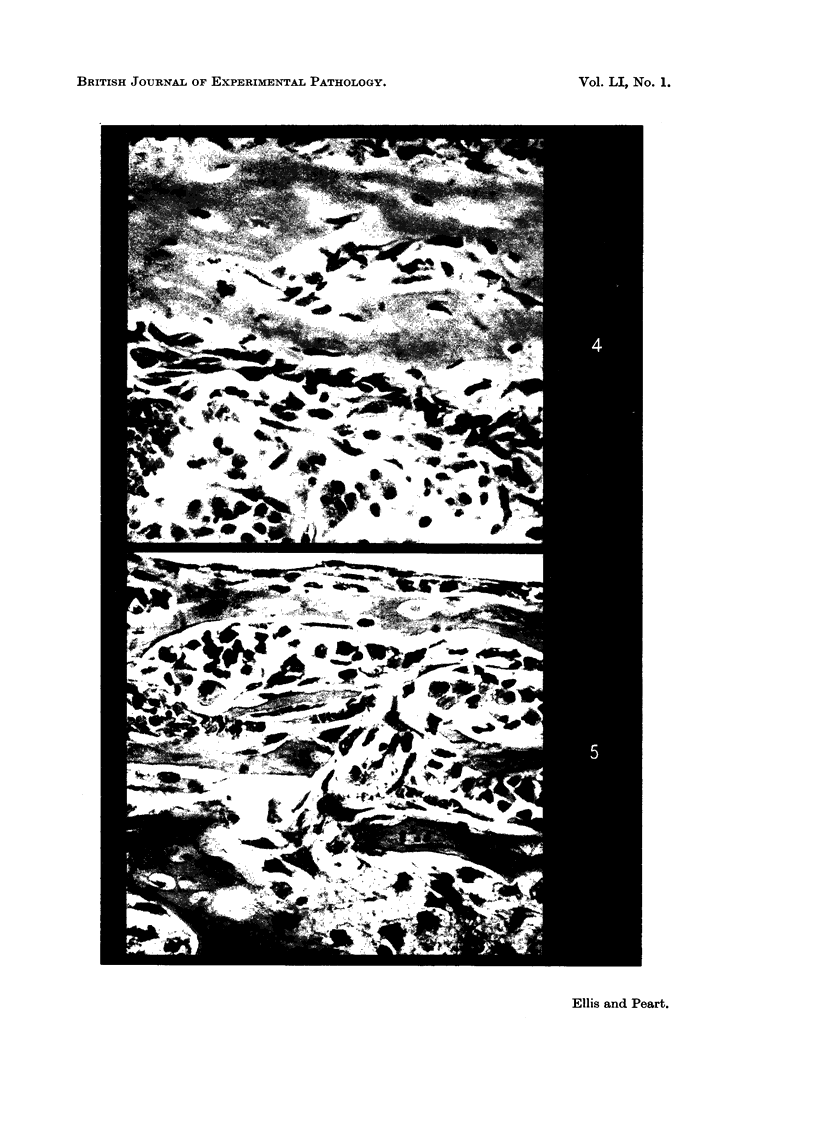
With 1·0 and 5·0 mg. per ml. of heparin or dextran sulphate there was increased resorption of bone and impaired new bone formation. At the highest concentration of 5 mg. per ml. both substances almost completely inhibited new bone formation. Undecalcified sections showed no loss of mineral from the remaining diaphyseal bone and there was no impairment of alkaline phosphatase activity demonstrated histochemically.
The concentrations of heparin or dextran sulphate required in the present tissue culture experiments to produce bone changes were higher than those achieved in the blood and tissue fluids of experimental animals even after prolonged administration. For the in vivo changes to be brought about by a direct effect of heparin or dextran sulphate on bone it would be necessary to postulate a selective accumulation of these substances in bone tissues.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS S. S., THORPE H. M., GLYNN L. E. Effect of a laminarin sulfphate (LM 46) on bone growth. Lancet. 1958 Sep 20;2(7047):618–620. doi: 10.1016/s0140-6736(58)90339-8. [DOI] [PubMed] [Google Scholar]
- ELLIS H. A., WALTON K. W. The estimation and recovery of dextran sulphates in biological fluids. J Clin Pathol. 1959 Sep;12:467–472. doi: 10.1136/jcp.12.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELL H. B., MELLANBY E. The effect of hypervitaminosis A on embryonic limb bones cultivated in vitro. J Physiol. 1952 Mar;116(3):320–349. doi: 10.1113/jphysiol.1952.sp004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELL H. B., WEISS L. THE EFFECT OF ANTISERUM, ALONE AND WITH HYDROCORTISONE, ON FOETAL MOUSE BONES IN CULTURE. J Exp Med. 1965 Apr 1;121:551–560. doi: 10.1084/jem.121.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLMAN T., NAIDOO S. S. In vitro effects of heparin and calcium ions on lipaemic serum. Nature. 1957 May 4;179(4566):904–905. doi: 10.1038/179904a0. [DOI] [PubMed] [Google Scholar]
- GOLDHABER P. HEPARIN ENHANCEMENT OF FACTORS STIMULATING BONE RESORPTION IN TISSUE CULTURE. Science. 1965 Jan 22;147(3656):407–408. doi: 10.1126/science.147.3656.407. [DOI] [PubMed] [Google Scholar]
- GOLDHABER P. The effect of hyperoxia on bone resorption in tissue culture. AMA Arch Pathol. 1958 Nov;66(5):635–641. [PubMed] [Google Scholar]
- HINT H. C., RICHTER A. W. Chronic toxicity of dextran sulphate in rabbits. Br J Pharmacol Chemother. 1958 Jun;13(2):109–112. doi: 10.1111/j.1476-5381.1958.tb00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAFFE M. D., WILLIS P. W., 3rd MULTIPLE FRACTURES ASSOCIATED WITH LONG-TERM SODIUM HEPARIN THERAPY. JAMA. 1965 Jul 12;193:158–160. doi: 10.1001/jama.1965.03090020072024. [DOI] [PubMed] [Google Scholar]
- JEAVONS S. M., RICKETTS C. R., WALTON K. W. Duration of anticoagulant effect in relation to urinary excretion of dextran sulphate. Br Med J. 1956 Nov 3;2(5000):1016–1023. doi: 10.1136/bmj.2.5000.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY L., PETRACEK F. J. Chemical and pharmacological studies on N-resulfated heparin. Proc Soc Exp Biol Med. 1962 Apr;109:901–905. doi: 10.3181/00379727-109-27372. [DOI] [PubMed] [Google Scholar]
- Lemaire A., Picard J., Gardais A. Le catabolisme de l'héparine. Etude in vivo de la désulfatation de l'héparine chez le rat. C R Acad Sci Hebd Seances Acad Sci D. 1967 Feb 13;264(7):949–952. [PubMed] [Google Scholar]
- McAllister B. M., Demis D. J. Heparin metabolism: isolation and characterization of uroheparin. Nature. 1966 Oct 15;212(5059):293–294. doi: 10.1038/212293a0. [DOI] [PubMed] [Google Scholar]
- RICKETTS C. R., WALTON K. W., SADDINGTON S. M. Preparation of dextran [35S] sulphate and tracer experiments in the rabbit. Biochem J. 1954 Dec;58(4):532–536. doi: 10.1042/bj0580532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWELL O. A. The culture of mature organs in a synthetic medium. Exp Cell Res. 1959 Jan;16(1):118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- WALTON K. W., ELLIS H. A., TAYLOR C. E. A method for the determination of the anticomplementary activity of heparin and related compounds. Br J Exp Pathol. 1957 Jun;38(3):237–247. [PMC free article] [PubMed] [Google Scholar]
- WEIGEL H., WALTON K. Synthesis of dextran sulphate labelled with carbon-14 and tracer experiments in the rat. Nature. 1959 Apr 4;183(4666):981–982. doi: 10.1038/183981a0. [DOI] [PubMed] [Google Scholar]