Abstract
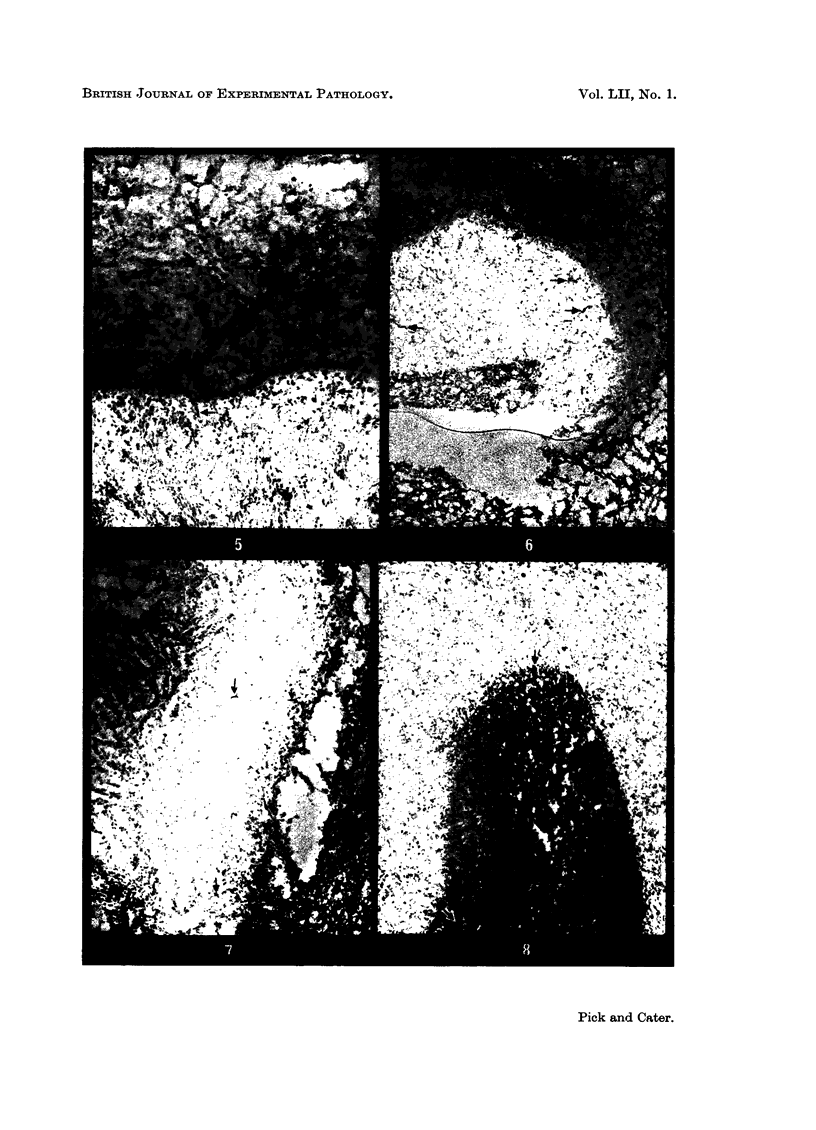
Fibrinolytic activity in tumours was studied by the fibrin slide technique. The tumour cells were inactive and fibrinolysis was seen only in areas with young blood vessels. In carrageenin-induced granulomas at 6 days the fibrinolytic activity was small and confined to mature veins, but from 7-14 days activity was high in zones containing young vessels supplying the terminal capillary buds; these latter showed no activity. In old fibrosed granulomas there was no fibrinolytic activity. The vascular permeability changes of inflammation (detected by the colloidal carbon technique) showed no correlation with fibrinolytic activity, and systemic injection of inflammatory agents had no effect on the fibrinolytic activity of the vessels. These findings are discussed in relationship to tumour vascularization.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrup T., Henrichsen J., Tympanidis K., King A. E. Fibrinolytically active vaginal epithelial cells. Nature. 1967 Apr 15;214(5085):297–298. doi: 10.1038/214297a0. [DOI] [PubMed] [Google Scholar]
- BENITZ K. F., HALL L. M. Local morphological response following a single subcutaneous injection of carrageenin in the rat. Proc Soc Exp Biol Med. 1959 Nov;102:442–445. doi: 10.3181/00379727-102-25278. [DOI] [PubMed] [Google Scholar]
- Back N., Shields R. R., DeWitt G., Branshaw R. H., Ambrus C. M. Uptake of fibrinogen and fibrinolytic enzymes by neoplastic tissue. J Natl Cancer Inst. 1966 Feb;36(2):171–180. [PubMed] [Google Scholar]
- Beneke G., Hey D. Modelluntersuchungen zur fermentativen Löslichkeit von Fibrin im histologischen Schnitt. Histochemie. 1965;5(5):366–377. doi: 10.1007/BF00306288. [DOI] [PubMed] [Google Scholar]
- CLIFFTON E. E., GROSSI C. E. Fibrinolytic activity of human tumors as measured by the fibrin-plate method. Cancer. 1955 Nov-Dec;8(6):1146–1154. doi: 10.1002/1097-0142(1955)8:6<1146::aid-cncr2820080610>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Cater D. B., Taylor C. R. Inflammatory changes in tumour vessels after systemic 5-hydroxytryptamine, bradykinin, kallikrein, or lysolecithin. Br J Cancer. 1966 Sep;20(3):517–525. doi: 10.1038/bjc.1966.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAY E. D., PLANINSEK J. A., PRESSMAN D. Localization in vivo of radio-iodinated anti-rat-fibrin antibodies and radio-iodinated rat fibrinogen in the Murphy rat lymphosarcoma and in other transplantable rat tumors. J Natl Cancer Inst. 1959 Feb;22(2):413–426. [PubMed] [Google Scholar]
- GROSSI C. E., AGOSTINO D., CLIFFTON E. E. The effect of human fibrinolysin on pulmonary metastases of Walker 256 carcinosarcoma. Cancer Res. 1960 Jun;20:605–608. [PubMed] [Google Scholar]
- HAWKINS W. W., LEONARD V. G. Antipeptic and antithrombic properties of carrageenin. J Lab Clin Med. 1962 Oct;60:641–648. [PubMed] [Google Scholar]
- HIRAMOTO R., BERNECKY J., JURANDOWSKI J., PRESSMAN D. Fibrin in human tumors. Cancer Res. 1960 Jun;20:592–593. [PubMed] [Google Scholar]
- Haustein U. F. Die Lokalisation des Gewebsaktivators der Fibrinolyse in Hauttumoren. Arch Klin Exp Dermatol. 1969;234(2):195–203. [PubMed] [Google Scholar]
- Henrichsen J., Astrup T. Fibrinolytically active rat vaginal epithelial cells. J Pathol Bacteriol. 1967 Apr;93(2):706–710. doi: 10.1002/path.1700930238. [DOI] [PubMed] [Google Scholar]
- LENDRUM A. C., FRASER D. S., SLIDDERS W., HENDERSON R. Studies on the character and staining of fibrin. J Clin Pathol. 1962 Sep;15:401–413. doi: 10.1136/jcp.15.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUGGLETON P. W., MACLAREN J. G., DYKE W. J. EFFECT OF PROTAMINE SULPHATE ON EXPERIMENTAL TUMOURS IN MICE. Lancet. 1964 Feb 22;1(7330):409–410. doi: 10.1016/s0140-6736(64)92788-6. [DOI] [PubMed] [Google Scholar]
- Montani E. The opacity variations of the plasma clot. Hemostase. 1966 May-Jun;6(2):185–197. [PubMed] [Google Scholar]
- O'MEARA R. A. Coagulative properties of cancers. Ir J Med Sci. 1958 Oct;394:474–479. [PubMed] [Google Scholar]
- O'MEARA R. A., THORNES R. D. Some properties of the cancer coagulative factor. Ir J Med Sci. 1961 Mar;423:106–112. doi: 10.1007/BF02952952. [DOI] [PubMed] [Google Scholar]
- Oswald N. T., Cater D. B. Effect of endotoxin from Serratia marcescens on the permeability of vessels in hepatomas and carrageenin granulomas of rats. Br J Exp Pathol. 1969 Feb;50(1):84–96. [PMC free article] [PubMed] [Google Scholar]
- SPAR I. L., BALE W. F., GOODLAND R. L., IZZO M. J. PREPARATION OF PURIFIED I-131-LABELED ANTIBODY WHICH REACTS WITH HUMAN FIBRIN. PRELIMINARY TRACER STUDIES ON TUMOR PATIENTS. Cancer Res. 1964 Feb;24:286–293. [PubMed] [Google Scholar]
- Schmidt-Matthiesen H. Uterus myomatosus als Aktivierungsherd einer chronischen, generalisierten Fibrinolyse. Geburtshilfe Frauenheilkd. 1966 Aug;26(8):1158–1165. [PubMed] [Google Scholar]
- THORNES R. D., MARTIN W. T. The cytopathic effect of fibrinolytic agents and human placental fractions on HeLa cells. Ir J Med Sci. 1961 Nov;431:487–494. doi: 10.1007/BF02953743. [DOI] [PubMed] [Google Scholar]
- THORNES R. D., O'MEARA R. A. A method of detecting and estimating inhibitors of the cancer coagulative factor. Ir J Med Sci. 1961 Aug;428:361–365. doi: 10.1007/BF02953365. [DOI] [PubMed] [Google Scholar]
- THORNES R. D., O'MEARA R. A. A method of detecting and estimating inhibitors of the cancer coagulative factor. Ir J Med Sci. 1961 Aug;428:361–365. doi: 10.1007/BF02953365. [DOI] [PubMed] [Google Scholar]
- Weiss G., Beller F. K. Localization of fibrinolytic activity in uterine cancer. Am J Obstet Gynecol. 1969 Apr 1;103(7):1023–1027. doi: 10.1016/s0002-9378(16)34454-4. [DOI] [PubMed] [Google Scholar]