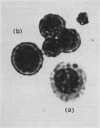
Abstract
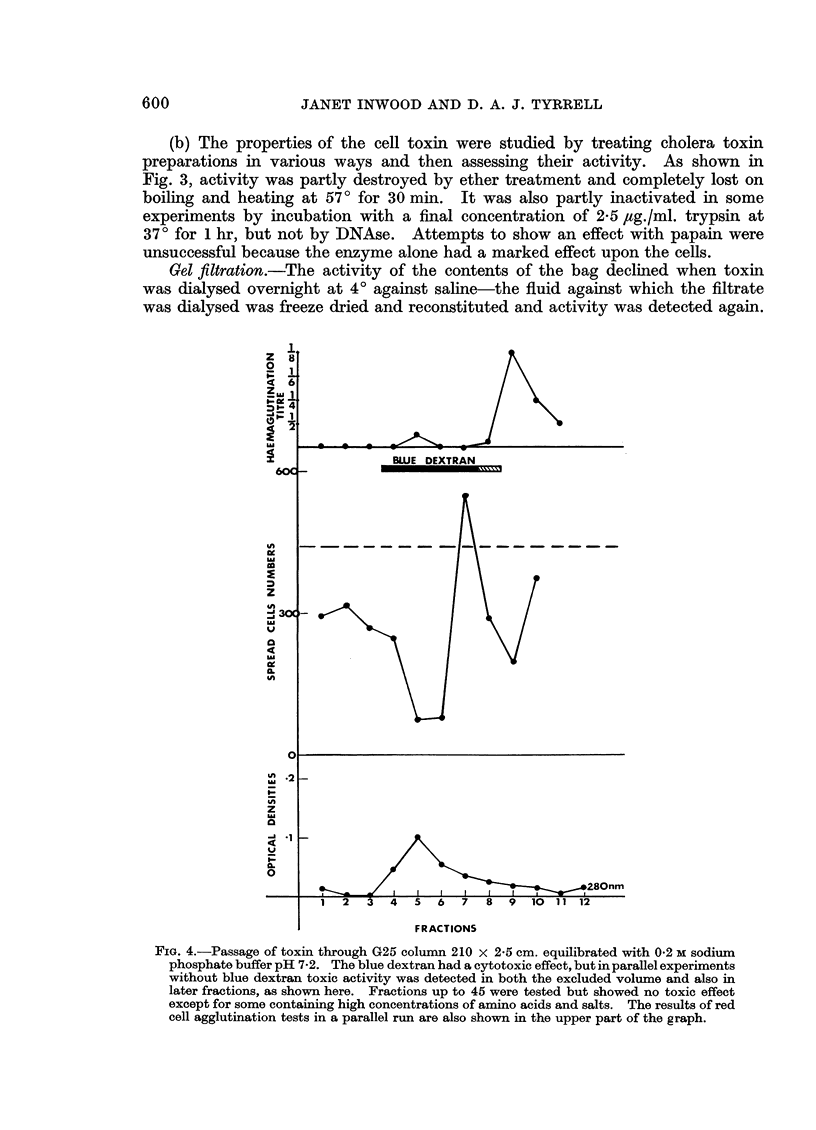
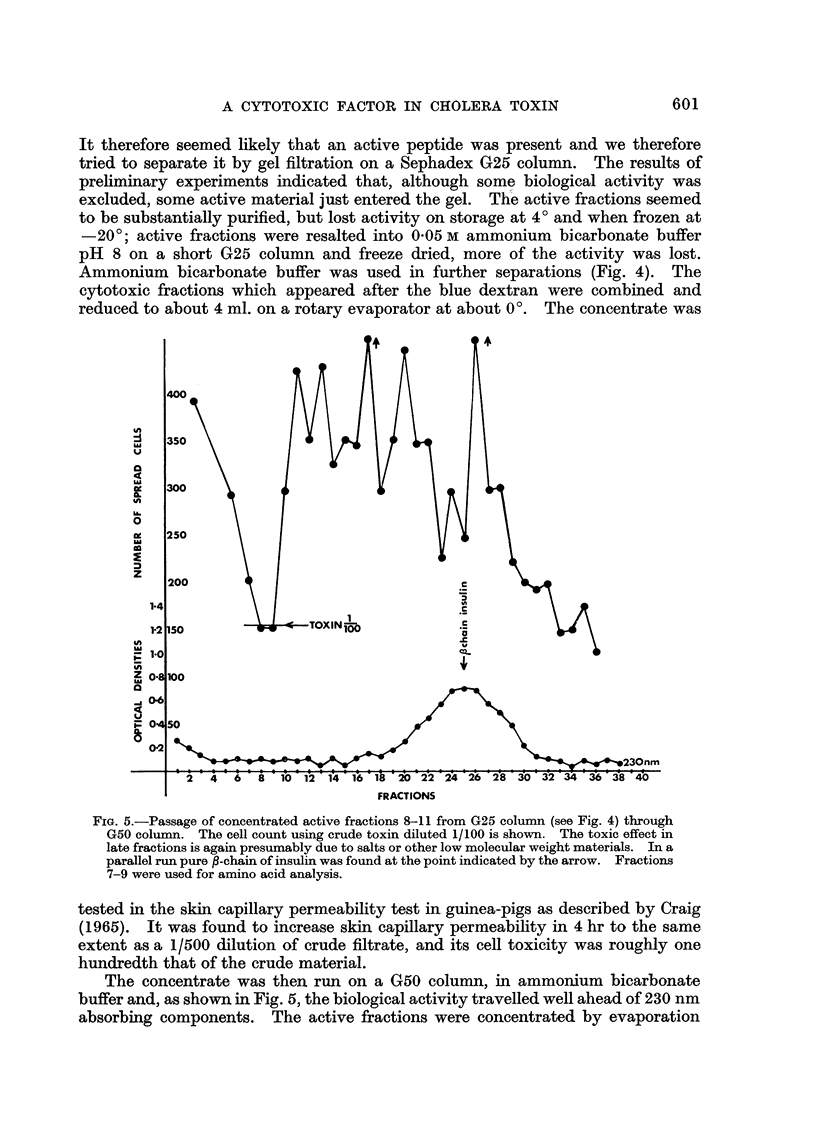
Preparations of cholera toxin prevented HeLa cells from spreading on a plastic surface. The toxic factor was heat labile. The toxin was filtered through Sephadex G25 and the cytotoxic component appeared to enter the resin, and cytotoxic fractions sensitized sheep cells to antitoxic serum. These fractions were then run on a G50 column and amino acid analysis suggested the presence in active fractions of a peptide containing 11 amino acids.
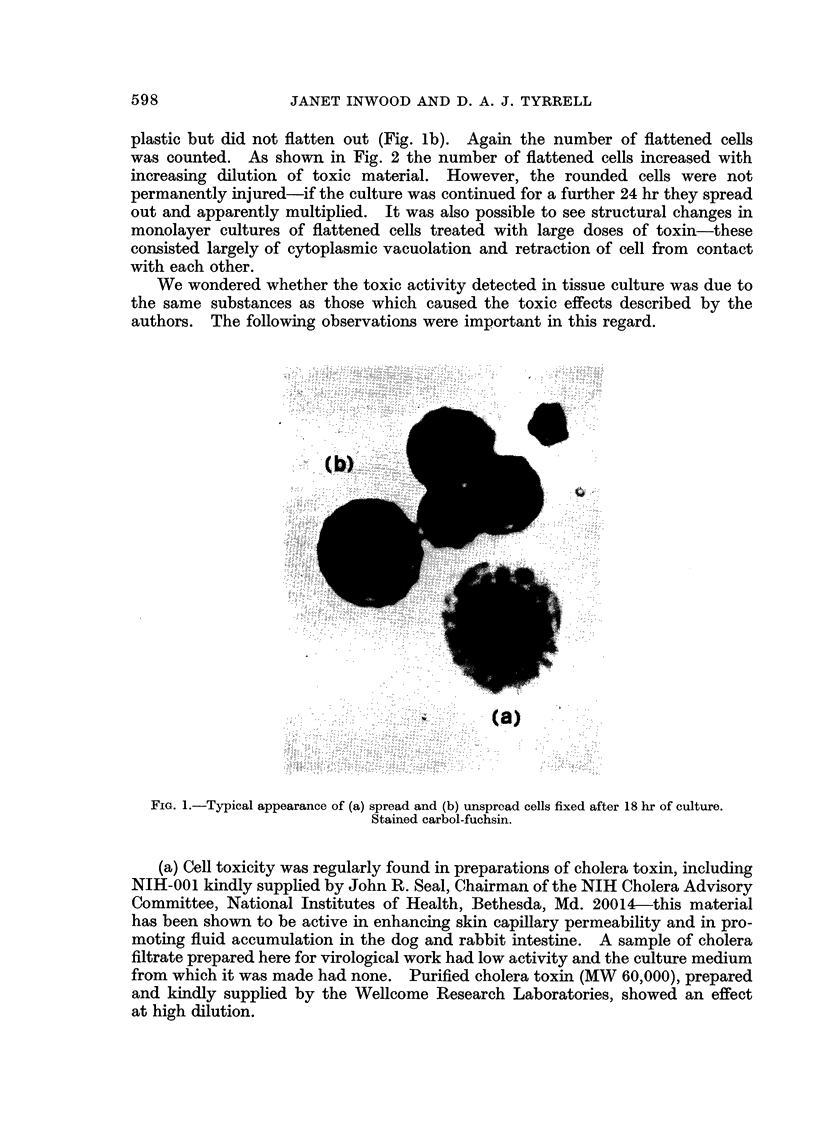
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein H. D., Feeley J. C., Richardson S. H. Titration of cholera antitoxin levels by passive hemagglutination tests using fresh and formalinized sheep erythrocytes. Proc Soc Exp Biol Med. 1970 Jan;133(1):120–124. doi: 10.3181/00379727-133-34421. [DOI] [PubMed] [Google Scholar]
- Kennedy J. R., Richardson S. H. Fine structure of Vibrio cholerae during toxin production. J Bacteriol. 1969 Dec;100(3):1393–1401. doi: 10.1128/jb.100.3.1393-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley W. H., Woodward W. E., Aziz K. M., Rahman A. S., Chowdhury A. K., Ahmed A., Feeley J. C. The 1968-1969 cholera-vaccine field trial in rural East Pakistan. Effectiveness of monovalent Ogawa and Inaba vaccines and a purified Inaba antigen, with comparative results of serological and animal protection tests. J Infect Dis. 1970 May;121(Suppl):1–9. doi: 10.1093/infdis/121.supplement.s1. [DOI] [PubMed] [Google Scholar]