Abstract
The ERF-1 transcription factor was previously shown to be involved in the regulation of estrogen receptor (ER) gene transcription in hormonally responsive breast and endometrial carcinomas. In this study we sought to identify the gene for ERF-1. ERF-1 activates ER gene transcription by binding to the imperfect palindrome CCCTGCGGGG within the promoter of the ER gene. ERF-1 protein was purified from the ER-positive breast carcinoma cell line, MCF7, utilizing ion exchange and DNA affinity chromatography. Peptide sequence analysis was used to isolate a 2.7 kb cDNA clone from an MCF7 cDNA library. This cDNA encodes a protein of 48 kDa previously identified as the AP2γ transcription factor. By gel-shift analysis, in vitro synthesized ERF-1 comigrates with MCF7 native ERF-1 complex and demonstrates identical sequence binding specificity as native ERF-1. In addition, AP2 polyclonal antisera supershifts both in vitro synthesized and native ERF-1 complexes. These results show that ERF-1 is a member of the AP2 family of developmentally regulated transcription factors. Given the central role of ER expression in breast carcinoma biology, ERF-1 is likely to regulate expression of a set of genes characteristic of the hormonally-responsive breast cancer phenotype.
Keywords: estrogen receptor, breast cancer, promoter, gene, RNA
Estrogen receptor (ER) expression plays a critical role in determining the prognosis and treatment of breast cancer (1). Patients with tumors that express ER have an improved survival and longer disease-free interval than patients with tumors lacking ER expression (2, 3). ER-positive tumors are more common in postmenopausal women and many of these tumors over-express ER as compared with ER levels in normal mammary epithelium (4). Tumors with abundant ER mRNA levels tend to be well-differentiated tumors which are PR-positive and have a lower nuclear grade as compared with tumors with low or absent ER mRNA (5, 6). It has been assumed that the phenotypic differences between hormonally responsive and unresponsive tumors is due to expression of genes regulated by ER (7). However, it is possible that ER is a marker for a well-differentiated tumor and ER may be only one of a set of genes whose expression is characteristic of the hormonally-responsive phenotype. Clearly, understanding the mechanisms that regulate ER gene expression will provide greater insight into the oncogenesis of breast carcinomas.
Previous studies have demonstrated that regulation of ER gene expression in ER-positive and ER-negative cell lines occurs at the level of ER gene transcription (8, 9). Some studies have suggested that gene methylation controls ER expression (9, 10). However, it is difficult to know whether methylation of the ER gene occurs as a mechanism controlling ER expression or is the result of a lack of transcriptional activity. We have previously performed a functional analysis of the human ER promoter (11). Utilizing a transient transfection assay, a 75 bp region of the 5′ untranslated leader of the ER gene was found to augment activity of the promoter. The ability of this region to augment expression was also found to be specific to ER-positive cell lines. Other studies of the mouse ER gene have also implicated the homologous region in determining proper tissue-specific expression (12). This region of the ER promoter was shown to bind a protein called ERF-1 (11). Using gel shift competitions, two ERF-1 binding sites were identified in this region of the ER promoter. Creation of point mutations in these two sites destroys ERF-1 binding and abolishes the ability for this region to augment promoter activity. In addition, abundant ERF-1 expression was only found in ER-positive breast and ER-positive endometrial carcinoma cell lines. Taken together, these data indicated a critical role for ERF-1 in ER gene regulation and suggested a common mechanism of ER transcriptional regulation in hormonally responsive cancers. To extend these studies of ERF-1, we sought to purify and characterize the ERF-1 transcription factor.
MATERIALS AND METHODS
Cell Lines.
MCF7 and MDA-MB-231 human breast carcinoma cell lines were obtained from American Type Culture Collection. Cells were maintained as previously described (11).
Gel Shift Assay.
Gel shift assays were performed as previously described (11) using 15 μg nuclear cell extract, purified chromatographic fractions or in vitro translated protein. In competitive binding assays, unlabeled mutant oligonucleotides were added at 1000 molar excess. Supershift assays were performed by the addition of 2 μg of affinity-purified rabbit polyclonal antibody directed against human AP2 (Santa Cruz Biochemicals) or with 2 μl of polyclonal La/SS-B antisera obtained as a gift from Michael Bachmann (Mainz, Germany). Probe was prepared by end-labeling the wild-type 30-bp double-stranded ERF-1 binding site with [γ-32P]ATP (3,000 Ci/mmol) using T4 polynucleotide kinase, followed by gel purification on 15% polyacrylamide. Twenty- or 30-bp oligonucleotides used as competitors were synthesized to contain double point mutations in relation to the wild-type sequence.
Purification of ERF-1.
MCF7 nuclei were prepared as previously described (13). Nuclei were resuspended in 0.6 times packed nuclear volume (PNV) of buffer D (10 mM Tris, pH 7.9/100 mM KCl/2 mM MgCl2/0.1 mM EDTA/1 mM DTT/1 mM NaMetaBis/0.2 mM phenylmethylsulfonyl fluoride) and Dounce homogenized on ice with 10 strokes. Homogenized nuclei were mixed with 0.06× PNV of 4 M ammonium sulfate (pH 7.9) and extracted with gentle mixing at 4°C for 1 h. Extracted nuclei were pelleted by centrifugation at 25,000 × g, 4°C for 20 min and the supernatant was dialyzed for 5.5 h against 5 liters of D-100 buffer (20 mM Hepes, pH 7.9/20% glycerol/100 mM KCl/2 mM MgCl2/0.2 mM EDTA/1 mM DTT/1 mM NaMetaBis/0.2 mM phenylmethylsulfonyl fluoride). After dialysis, the extract was centrifuged for 20 min at 16,000 × g, 4°C. Protein concentration of nuclear extract was determined using a Bio-Rad protein assay system (Bio-Rad) and was generally in the range of 7–15 mg/ml.
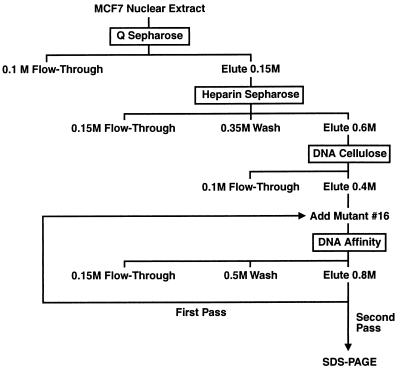
ERF-1 activity was monitored throughout the purification by gel-shift assay. MCF7 nuclear extract (375 mg) was applied to a 30 ml Q Sepharose Fast Flow (Pharmacia) anion exchange column at 100 mM KCl and eluted with 1 volume of 0.15 M KCl. This fraction was applied to a 10 ml Heparin Sepharose CL-6B (Pharmacia) column, washed with 5 volumes of 0.35 M KCl and eluted with 2.5 volumes of 0.6 M KCl. This 0.6 M fraction was diluted to 0.1 M KCl, applied to a 5 ml DNA cellulose (native DNA; Pharmacia) column, and eluted with 2.5 volumes of 0.4 M KCl. This fraction was diluted to 0.15 M KCl and nonbinding mutant 16 (TGAGCCTTCTGCGGTGCGGGGACACCGTCT) was added at 10 μg/ml, incubated on ice 10 min and centrifuged at 12,000 × g for 10 min at 4°C to clear any precipitated material. The protein was divided in half and applied to two 1 ml DNA affinity columns. The columns were washed with 5 volumes of 0.5 M KCl and eluted with 5 volumes of 0.8 M KCl. The ERF-1 fraction was incubated with additional mutant oligonucleotide (10 μg/ml) and passed a second time over a single 1 ml DNA affinity column as described above. All fractions were quick frozen in liquid nitrogen and stored at −80°C for subsequent analysis. The DNA affinity column was prepared by attaching a double-stranded, biotinylated 30-mer oligonucleotide (TGAGCCTTCTGCCCTGCGGGGACACGGTCT) corresponding to the wild-type ERF-1 binding site to streptavidin agarose.
Protein fractions were concentrated by trichloroacetic acid precipitation as described (14). Protein pellets were resuspended in standard Laemmli SDS loading buffer, boiled 3 min, and electrophoresed on 8% SDS/PAGE with standard protein molecular weight markers. Proteins were visualized using the Silver Stain Plus kit (Bio-Rad).
Protein Sequencing.
Protein was separated by SDS/PAGE, stained with Coomassie brilliant blue, and individual protein bands excised. After in gel S-carboxyamidomethylation, the bands were subjected to in gel tryptic (Promega) digestion as described (15) without the addition of 0.02% Tween. Candidate ions for sequencing were determined by microcapillary high performance liquid chromatography coupled to a triple mass spectrometer (model TSQ7000 with electrospray ionization source; Finnigan, San Jose, CA) as described (16). The ion densities observed corresponded to a load of 40–100 fmol by comparison with average ion abundance of a BSA standard protein digest. Direct peptide sequence information was obtained by collisionally induced dissociation on an equivalent injection of the digest mixture. The resulting MS/MS spectra were manually interpreted. The database searching algorithm sequest (17) was also used to facilitate interpretation of MS/MS spectra.
UV Crosslinking.
DNA/protein crosslinking of the ERF-1 complex was performed as described (18) using 15 μg of MCF7 nuclear extract. Radiolabeled probe was prepared as described by annealing 1 pmol of an oligonucleotide encompassing the ERF-1 binding site (CCGTGTCCCCGCAGGGCAGAAGGCTCA) (Operon Technologies) with 100 pmol of a complimentary oligonucleotide (TGAGCCTTCT) (Ana-Gen Technologies).
Renaturation of ERF-1 Activity from SDS/PAGE.
Purified extract was resolved on 8% SDS/PAGE after which the gel was segmented into 4 mm slices ranging from 31 to 220 kDa. Each slice was crushed in 0.4 ml elution buffer (0.15 M NaCl/0.1% SDS/0.05 M Tris⋅HCl, pH 7.9/0.1 mM EDTA/5 mM DTT/0.1 mg/ml BSA) and protein was eluted for 1 h at room temperature, followed by precipitation with 4 volumes of cold acetone. The pellet was washed with cold 80% acetone. Precipitated protein was dissolved in 0.5 ml of 8 M urea in D-100 buffer and incubated at 4°C for 30 min. The protein was renatured by dialysis against 1 liter of 1 M urea in D-100 buffer for 3 h, followed by two changes of D-100 buffer for 3 h and 12 h, respectively.
Cloning of ERF-1 from an MCF7 cDNA Library.
Primers AACCCTCTGAACCTC CCCTGTCAGAAG and CCGGTCTTGGCTGAGAAGTTCTGTGAATTC (Operon Technologies) were used to amplify a 504-bp fragment of the ERF-1 mRNA from MCF7 using the GeneAmp RNA PCR kit (Perkin–Elmer). This fragment was random primed labeled using [α-32P]dCTP for use as a probe to retrieve a full-length ERF-1 cDNA clone from an MCF7 expression library prepared using the Zap Express cDNA Synthesis kit (Stratagene). Clones were excised from λ phage as phagemids and were analyzed by restriction analysis. ERF-1 cDNA was sequenced using an Applied Biosystems automated sequencer.
In Vitro Transcription and Translation.
ERF-1 was in vitro translated from ERF-1 cDNA using the TnT Coupled Reticulocyte Lysate System (Promega). Unlabeled ERF-1 was translated in vitro by using a complete amino acid mix in place of [35S]methionine. For immunoprecipitations, 15 μl of in vitro translated ERF-1 protein was incubated on ice for 10 min with 2 μg of affinity-purified rabbit polyclonal AP2 antibody in a 200 μl volume of RIPA buffer (150 mM NaCl/50 mM Tris, pH 8.0/1% Nonidet 40/0.5% deoxycholate/0.1% SDS). Fifty microliters of anti-rabbit IgG (whole molecule) agarose (Sigma) was added to the reaction and incubated on ice for 15 min. Immunoprecipitated protein was pelleted by centrifugation at 1,000 × g at 4°C for 5 min, washed once with RIPA buffer and twice each with buffer 1 (150 mM NaCl/10 mM Tris, pH 7.5/2 mM EDTA/1% Nonidet P-40) and buffer 2 (500 mM NaCl/10 mM Tris, pH 7.5/2 mM EDTA), and the final pellet was resuspended in standard Laemmli SDS loading buffer for SDS/PAGE analysis.
RESULTS
Mutational Analysis of the ERF-1 Site.
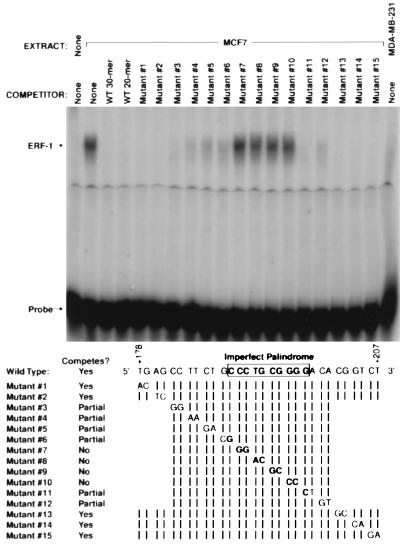
A 30 bp double stranded DNA probe was used in gel shift assays for the identification and purification of ERF-1. This probe is composed of DNA sequences between +178 and +207 from the untranslated leader of the ER gene promoter. This sequence contains the imperfect palindrome, CCCTGCGGGG, previously defined as a high-affinity ERF-1 binding site. Gel-shift competition using a series of mutants in this region is shown in Fig. 1. Mutants 7, 8, 9, and 10 disrupt the imperfect palindrome and fail to compete for binding to the wild-type probe. Mutations that target several nucleotides flanking the palindrome demonstrate partial binding specificity. The 30 bp mutants 1, 2, 13, 14, and 15 demonstrate competition equal to wild-type probe. These results confirm that the ERF-1 binding site is represented by the imperfect palindrome with some sequence specificity demonstrated by flanking nucleotides. The ER-negative cell line, MDA-MB-231, does not make detectable ERF-1.
Figure 1.
Mutational analysis of ERF-1 binding site. Gel shift analysis using nuclear extract prepared from MCF7 cells (lanes 2–19) or MDA-MB-231 (lane 20) with 30-bp wild-type oligonucleotide as probe. Competition with mutant oligonucleotides as listed.
Purification of ERF-1.
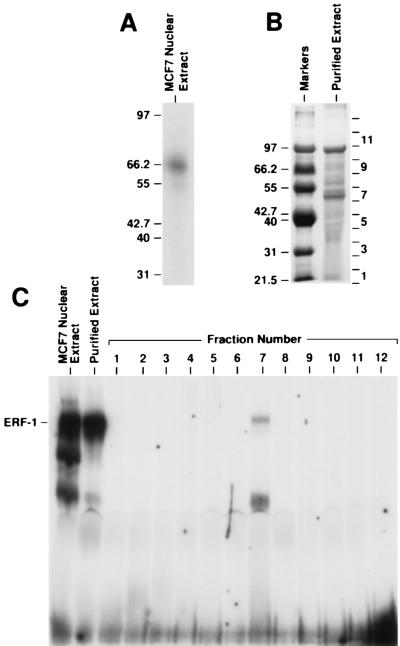
ERF-1 protein was purified from MCF7 nuclear extract using ion exchange and DNA affinity chromatography. A diagram of the purification protocol is shown in Fig. 2. ERF-1 activity was followed through the purification process using gel-shift analysis. Both UV crosslinking and gel renaturation experiments indicated the size of ERF-1 as ≈50 kDa. First, the bound ERF-1 complex resolved by gel shift was UV crosslinked to a radiolabeled ERF-1 DNA probe. This protein–DNA complex had an apparent molecular weight of 60–65 kDa on SDS/PAGE as shown in Fig. 3A. Subtracting the molecular weight of the DNA would indicate a protein of ≈50–55 kDa. Second, ERF-1 activity was renatured from SDS/PAGE. Purified extract was electrophoresed on 8% SDS/PAGE as shown in Fig. 3B. This purified extract contained two major bands, as well as several minor species. The gel was sliced into 12 separate sections, the protein renatured and analyzed by gel-shift. As can be seen in Fig. 3C, fraction number 7 contains ERF-1 activity. This renatured complex comigrates with native ERF-1 complex. It has also been shown that this renatured complex demonstrates identical binding specificity as determined for the native protein as shown in Fig. 1 (data not shown). All other fractions are negative for binding activity. Fraction number 7 corresponds to a region of the gel of ≈50 kDa, which agrees with the size determined by UV crosslinking studies.
Figure 2.
Purification of ERF-1. Protocol for purification of ERF-1 protein using ion exchange and DNA affinity chromatography. See text for details.
Figure 3.
Size determination of ERF-1. (A) ERF-1 complex was resolved by gel-shift analysis and the gel was subjected to UV crosslinking. The bound complex was excised and resolved on SDS/PAGE as shown. The location of molecular weight markers (kDa) is shown to the left of the gel lane. (B) Purified extract was electrophoresed on 8% SDS/PAGE and sectioned into 12 gel slices. The approximate location of gel slices 1–12 are shown to the right of the gel lane. Molecular weight markers (kDa) are listed to the left of the lane labeled markers. (C) Gel shift analysis of proteins renatured from gel slices in B. Fraction number 7 demonstrated ERF-1 activity.
Fraction number 7 of the purified extract contained two obvious protein bands. Each of these bands was excised and subjected to peptide sequencing. The lower, more prominent band was identified as La/SS-B, which is an RNA binding protein (19). La/SS-B was unlikely to be ERF-1 based upon known characteristics of this protein. In addition, three separate antibodies to La/SS-B failed to supershift native ERF-1 complex (data not shown). Sequence analysis of the upper, less prominent band yielded two peptide sequences, NPLNLPCQK and EFTELLSQDR. Both of these peptides matched precisely the amino acid sequence of a partial cDNA clone for AP2γ (GenBank accession no. X95693X95693). AP2γ was identified based upon homology to the AP2α transcription factor (20). Two DNA oligonucleotide primers were prepared corresponding to the two peptide sequences obtained from peptide sequence analysis. These primers were used to amplify MCF7 mRNA utilizing RT-PCR, which generated an expected PCR product of 504 bp (data not shown). This PCR product was used as a probe to screen an MCF7 cDNA library. Thirteen separate cDNA clones were obtained, with the nine largest clones having an identical pattern on restriction analysis. The size of these cDNA inserts was 2.7 kb. One of these cDNAs was sequenced completely and this sequence is shown in Fig. 4A. This cDNA contains a long open reading frame of 1353 bp encoding a protein with a predicted molecular weight of 48 kDa. The predicted amino acid sequence of the ERF-1 protein matched precisely the amino acid sequence reported for AP2γ. The ERF-1 cDNA contains a 166-bp 5′ untranslated leader and a 1268-bp 3′ untranslated region. Alignment of the ERF-1 protein with AP2α is shown in Fig. 4B. Overall, there is 65% identity and 83% similarity between these two proteins. Homology is most striking within the carboxyl-terminal half of the proteins which contains the DNA binding and dimerization domains. Within the carboxyl-terminal half there is 76% identity and 90% similarity between these two proteins.
Figure 4.
ERF-1 cDNA. (A) Complete sequence of ERF-1 cDNA with predicted amino acid sequence. Boxes indicate peptide sequences obtained from protein sequencing of purified ERF-1. (B) Comparison of ERF-1 protein sequence with AP2α protein aligned to highlight similarities. ∗, Identical amino acids; ⋅ conserved substitution.
Confirmation of ERF-1 Identity.
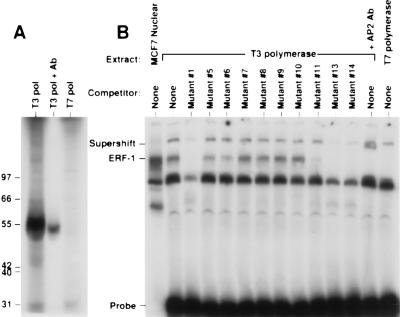
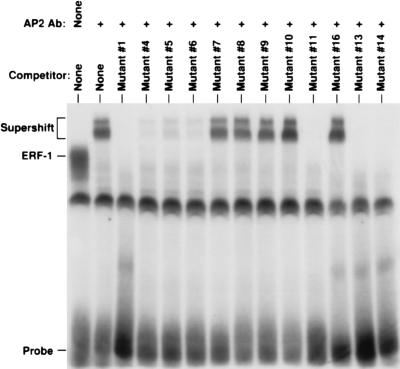
The identity of ERF-1 was confirmed by analysis of in vitro synthesized product and by antibody reactivity. The ERF-1 cDNA was subjected to in vitro transcription/translation. As can be seen in Fig. 5A, the in vitro product has a molecular weight of ≈50 kDa. A polyclonal antisera generated against the carboxyl-terminal 20 amino acids of AP2α is able to immunoprecipitate the in vitro synthesized product. The T7 promoter is directed in the opposite orientation and, as expected, no product is obtained. In vitro synthesized ERF-1 was prepared in the absence of 35S-labeled amino acids. This cold protein was analyzed in a gel-shift assay as shown in Fig. 5B. In vitro synthesized ERF-1 generates an ERF-1 complex that co-migrates with the native ERF-1 complex. This product also has the same DNA sequence specificity as demonstrated with competition using mutant oligonucleotide sequences. In addition, AP2α antisera supershifts the in vitro ERF-1 complex. As expected, the extract prepared using the T7 polymerase does not generate the ERF-1 complex. The AP2α antisera should also identify the native ERF-1 complex. As can be seen in Fig. 6, this antisera supershifts all of the ERF-1 complex from MCF7 cells. This supershifted complex also demonstrates the same pattern of sequence binding as determined by mutant competitors.
Figure 5.
In vitro synthesized ERF-1. (A) In vitro transcription/translation from the ERF-1 cDNA using T3 polymerase (sense) (lane 1) or T7 polymerase (antisense) (lane 3). In vitro synthesized ERF-1 was immunoprecipitated using AP2 polyclonal antisera (lane 2). (B) Gel shift analysis of in vitro synthesized ERF-1 (lanes 2–13) with mutant competitors compared with MCF7 nuclear extract (lane 1). In vitro ERF-1 complex supershifted with AP2 antisera (lane 13).
Figure 6.
Native ERF-1 complex supershift. MCF7 nuclear extract analyzed by gel shift with AP2 antisera (lanes 2–14) with competitors as shown compared with native ERF-1 complex (lane 1).
It is evident that the ERF-1 binding sequence is similar to the sequence specificity of AP2α (GCCNNNGGC) (21) and AP2α antisera cross reacts with ERF-1 protein. Given the extensive homology between AP2 family members, it might be questioned whether the ERF-1 complex is formed by AP2α homodimers. However, several considerations make this possibility unlikely. First, HeLa cells express AP2α whereas ERF-1 activity was not readily demonstrated in this cell line and several breast carcinoma cell lines known to express ERF-1 do not express AP2α (11, 22). Second, although cloned AP2α protein binds to the wild-type ERF-1 probe by gel shift analysis, the complex formed demonstrates different binding specificities for mutant competitors and has slightly faster mobility as compared with native ERF-1 complex (data not shown). Finally, the peptides obtained from sequencing purified ERF-1 differ from the amino acid sequence for AP2α. These findings indicate that ERF-1, previously shown to be involved in regulation of the ER gene promoter, is an AP2 family member distinct from AP2α.
DISCUSSION
A functional analysis of the ER promoter in breast carcinoma cells identified the ERF-1 transcription factor and established a role for ERF-1 in ER gene regulation in hormonally responsive carcinomas (11). We have now cloned the gene for ERF-1 and have established that ERF-1 is a member of the AP2 transcription factor family. Three members of this family have been identified, AP2α [originally called AP2 (21)], AP2β and AP2γ (20). The protein sequence for ERF-1 matches precisely the predicted amino acid sequence for AP2γ. The identification of ERF-1 as a member of a well-characterized transcription factor family provides additional proof supporting the role of ERF-1 in ER gene regulation. However, it remains to be determined if ERF-1 is responsible for basal tissue-specific expression of ER or if ERF-1 induces the over-expression of ER that is characteristic of many ER-positive carcinomas. Identification of the ERF-1 gene now facilitates the resolution of these mechanistic issues.
The homology of ERF-1 with AP2α raises several points of speculation concerning the function and biology of ERF-1. AP2α activates transcription by binding as a homodimer to specific DNA sequences (21). We presume that ERF-1 will also form homodimers but this possibility has not been formally tested. However, heterodimer formation between ERF-1 and other members of the AP2 family may also be possible, raising an additional potential for complex gene regulation. Heterodimer formation between murine AP2α and AP2β has previously been reported (23). The formation of heterodimers may alter normal function of AP2 proteins or, alternatively, may generate complexes with different specificity. Germane to gene regulation in breast carcinomas, recent reports have implicated AP2 factors in the transcriptional control of c-erbB-2 (22), E-cadherin (24) and HSP27 (25). It is certainly plausible to hypothesize a role for ERF-1 in the coordinate regulation of a set of genes in hormonally responsive carcinomas. This conjecture is supported by the fact that breast carcinoma cell lines that express E-cadherin and HSP27 are also ERF-1 positive, whereas cell lines with low level expression of these genes are ERF-1 negative (11, 24, 25). Identifying other genes regulated by ERF-1 will be an important area for further study.
A previous study localized the gene for ERF-1 to chromosome 20q13.2 (20). Chromosomal amplification of 20q13.2 has been demonstrated in the MCF7 cell line (26). Studies of primary breast tumors have identified a >1.5-fold amplification of 20q13.2 in 41 of 146 tumors (28%) and >3-fold amplification in 9.6% of tumors (27). Given the role of ERF-1 in ER gene expression, it is tempting to postulate that ERF-1 is the critical gene selecting for amplification of this region of chromosome 20 in breast cancers.
Acknowledgments
We thank Dr. Joseph R. Nevins (Duke University Medical Center, Durham, NC) for providing scientific insight during the purification of ERF-1 and for critical review of the manuscript. We also thank William S. Lane and Theresa A. Addona (Harvard Microchemistry Facility) for expertise in HPLC and microsequencing by tandem mass spectrometry. This work was supported in part by National Institutes of Health Grant R29 CA63251 and U.S. Department of the Army Medical Research and Development Command Grant DAMD17-94-J-4353. L.A.M. was funded through the University of California Breast Cancer Research Project Grant 1FB-0377. R.J.W. was funded in part by a fellowship from the American Surgical Association and a Clowes Career Development Award through the American College of Surgeons.
ABBREVIATION
- ER
estrogen receptor
Footnotes
References
- 1.Iglehart J D. In: Textbook of Surgery. Sabiston D C, editor. Philadelphia: Saunders; 1991. pp. 510–550. [Google Scholar]
- 2.Knight W A, III, Livingston R B, Gregory E J, McGuire W L. Cancer Res. 1977;37:4669–4671. [PubMed] [Google Scholar]
- 3.Sigurdsson H, Baldetorp B, Borg Å, Dalberg M, Fernö M, Killander D, Olsson H. N Engl J Med. 1990;322:1045–1053. doi: 10.1056/NEJM199004123221505. [DOI] [PubMed] [Google Scholar]
- 4.Ricketts D, Turnbull L, Ryall G, Bakhshi R, Rawson N S B, Gazet J-C, Nolan C, Coombes R C. Cancer Res. 1991;51:1817–1822. [PubMed] [Google Scholar]
- 5.Henry J A, Nicholson S, Farndon J R, Westley B R, May F E B. Br J Cancer. 1988;58:600–605. doi: 10.1038/bjc.1988.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeci, C., deConinck, E. C., Lawton, T., Bloch, D. A. & Weigel, R. J. (1997) Am. J. Pathol., in press. [PMC free article] [PubMed]
- 7.Fuqua S A W, Chamness G C, McGuire W L. J Cell Biochem. 1993;51:135–139. doi: 10.1002/jcb.240510204. [DOI] [PubMed] [Google Scholar]
- 8.Weigel R J, deConinck E C. Cancer Res. 1993;53:3472–3474. [PubMed] [Google Scholar]
- 9.Ottaviano Y L, Issa J-P, Parl F F, Smith H S, Baylin S B, Davidson N E. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- 10.Lapidus R G, Ferguson A T, Ottaviano Y L, Parl F F, Smith H S, Weitzman S A, Baylin S B, Issa J-P J, Davidson N E. Clin Cancer Res. 1996;2:805–810. [PubMed] [Google Scholar]
- 11.deConinck E D, McPherson L A, Weigel R J. Mol Cell Biol. 1995;15:2191–2196. doi: 10.1128/mcb.15.4.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicatiello L, Cobellis G, Addeo R, Papa M, Altucci L, Sica V, Bresciani F, LeMeur M, Kumar V L, Chambon P, Weisz A. Mol Endocrinol. 1995;9:1077–1090. doi: 10.1210/mend.9.8.7476981. [DOI] [PubMed] [Google Scholar]
- 13.Digham J D, Lebovitz R M, Roeder R G. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson G L. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 15.Hellman U, Wernstedt C, Goñez J, Heldin C-H. Anal Biochem. 1995;191:332–336. doi: 10.1006/abio.1995.1070. [DOI] [PubMed] [Google Scholar]
- 16.Hunt D F, Henderson R A, Shabanowitz J, Sakaguchi K, Michel K, Sevilir N, Cox A, Appella E, Engelhard V H. Science. 1992;255:1261. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 17.Eng J K, McCormick A L, Yates J R., III J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 18.Ballard D W, Böhnlein E, Hoffman J A, Bogerd H P, Dixon E P, Franza B R, Greene W C. New Biol. 1989;1:83–92. [PubMed] [Google Scholar]
- 19.Bachman M, Pfeifer K, Schröder H C, Müller W E G. Cell. 1990;60:85–93. doi: 10.1016/0092-8674(90)90718-t. [DOI] [PubMed] [Google Scholar]
- 20.Williamson J A, Bosher J M, Skinner A, Sheer D, Williams T, Hurst H C. Genomics. 1996;35:262–264. doi: 10.1006/geno.1996.0351. [DOI] [PubMed] [Google Scholar]
- 21.Williams T, Tjian R. Genes Dev. 1991;5:670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- 22.Bosher J M, Williams T, Hurst H C. Proc Natl Acad Sci USA. 1995;92:744–747. doi: 10.1073/pnas.92.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moser M, Imhof A, Pscherer A, Bauer R, Amselgruber W, Sinowatz F, Hofstädter F, Schüle R, Buettner R. Development (Cambridge, UK) 1995;121:2779–2788. doi: 10.1242/dev.121.9.2779. [DOI] [PubMed] [Google Scholar]
- 24.Hennig G, Löwrick O, Birchmeier, Behrens J. J Biol Chem. 1996;271:595–602. doi: 10.1074/jbc.271.1.595. [DOI] [PubMed] [Google Scholar]
- 25.Oesterreich S, Hickey E, Weber L A, Fuqua S A W. Biochem Biophys Res Commun. 1996;222:155–163. doi: 10.1006/bbrc.1996.0714. [DOI] [PubMed] [Google Scholar]
- 26.Tanner M M, Tirkkonen M, Kallioniemi A, Collins C, Stokke T, Karhu R, Kowbel D, Shadravan F, Hintz M, Kuo W-L, Waldman F M, Isola J J, Gray J W. Cancer Res. 1994;54:4257–4260. [PubMed] [Google Scholar]
- 27.Tanner M M, Tirkkonen M, Kallioniemi A, Isola J, Kuukasjärvi T, Collins C, Kowbel D, Guan X-Y, Trent J, Gray J W, Meltzer P, Kallioiniemi O-P. Cancer Res. 1996;56:3441–3445. [PubMed] [Google Scholar]