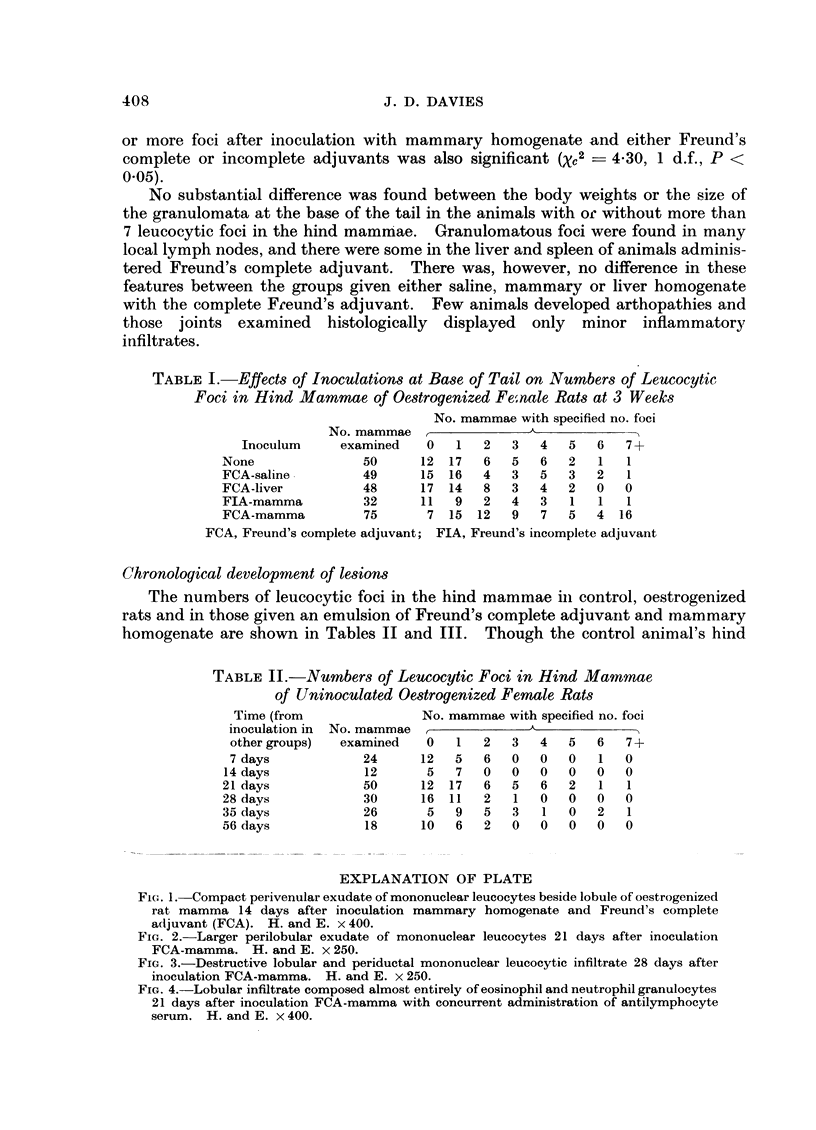
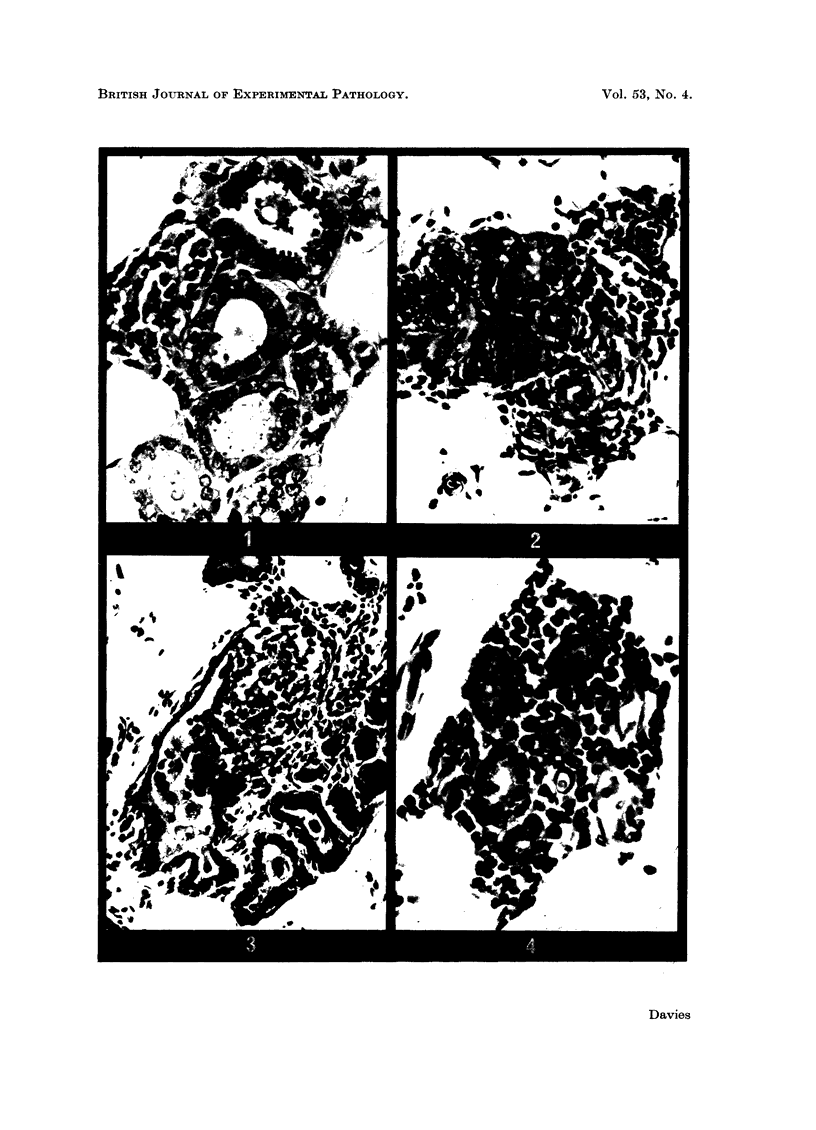
Abstract
Inoculation of 0·1 ml of an emulsion of a saline suspension of allogeneic lactating mammae and Freund's complete adjuvant into oestrone-primed female Wistar rats evoked in their mammary glands a progressively increasing focal mononuclear leucocytic infiltrate that was found in 25·0% of the animals 28 days after inoculation. Comparable changes were not seen during the experimental period in rats administered oestrone alone, or in those inoculated with other combinations of saline, lactating mammary or liver homogenates and Freund's complete or incomplete adjuvants. The mononuclear inflammatory lesions were substantially unaffected by the site of the intradermal inoculations, or by the addition of pertussis vaccine to the inocula, but were suppressed by antilymphocyte serum. It is suggested that some examples of human periductal mastitis may have an autoallergic basis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FLAX M. H. Experimental allergic thyroiditis in the guinea pig. II. Morphologic studies of development of disease. Lab Invest. 1963 Feb;12:199–213. [PubMed] [Google Scholar]
- FREUND J., LIPTON M. M., THOMPSON G. E. Aspermatogenesis in the guinea pig induced by testicular tissue and adjuvants. J Exp Med. 1953 May;97(5):711–726. doi: 10.1084/jem.97.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUND J. Sensitization with organ specific antigens and the mechanism of enhancement of the immune responses. J Allergy. 1957 Jan;28(1):18–29. doi: 10.1016/0021-8707(57)90067-9. [DOI] [PubMed] [Google Scholar]
- Foote F. W., Stewart F. W. Comparative Studies of Cancerous Versus Noncancerous Breasts. Ann Surg. 1945 Jan;121(1):6–53. doi: 10.1097/00000658-194501000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES H. E., ROITT I. M. Experimental auto-immune thyroiditis in the rat. Br J Exp Pathol. 1961 Dec;42:546–557. [PMC free article] [PubMed] [Google Scholar]
- Kalden J., James K. K., Williamson W. G., Irvine W. J. The suppression of experimental thyroiditis in the rat by heterologous anti-lymphocyte globulin. Clin Exp Immunol. 1968 Nov;3(9):973–981. [PMC free article] [PubMed] [Google Scholar]
- LERNER E. M., 2nd, MCMASTER P. R., EXUM E. D. THE COURSE OF EXPERIMENTAL AUTOALLERGIC THYROIDITIS IN INBRED GUINEA PIGS. THE PATHOLOGIC CHANGES AND THEIR RELATIONSHIP TO THE IMMUNE RESPONSE OVER A 2 YEAR PERIOD. J Exp Med. 1964 Feb 1;119:327–342. doi: 10.1084/jem.119.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE S., WENK E. J. A HYPERACUTE FORM OF ALLERGIC ENCEPHALOMYELITIS. Am J Pathol. 1965 Jul;47:61–88. [PMC free article] [PubMed] [Google Scholar]
- LEVINE S., WENK E. J. ALLERGIC ENCEPHALOMYELITIS: A HYPERACUTE FORM. Science. 1964 Dec 25;146(3652):1681–1682. doi: 10.1126/science.146.3652.1681. [DOI] [PubMed] [Google Scholar]
- Levey R. H., Medawar P. B. Nature and mode of action of antilymphocytic antiserum. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1130–1137. doi: 10.1073/pnas.56.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER M. N., KAPPAS A. ESTROGEN PHARMACOLOGY. II. SUPPRESSION OF EXPERIMENTAL IMMUNE POLYARTHRITIS. Proc Soc Exp Biol Med. 1964 Dec;117:845–847. doi: 10.3181/00379727-117-29715. [DOI] [PubMed] [Google Scholar]
- Macsween R. N., Ono K., Bell P. R., Thomason C. M., Starzl T. E. Experimental allergic thyroiditis in rats: suppression by heterologous (rabbit) anti-lymphocyte sera to lymph node, thymic and splenic lymphocytes. Clin Exp Immunol. 1970 Feb;6(2):273–278. [PMC free article] [PubMed] [Google Scholar]
- Paterson P. Y., Drobish D. G. Rapid induction of experimental allergic thyroiditis in rats sensitized to thyroid-adjuvant and pertussis vaccine. J Immunol. 1968 Nov;101(5):1098–1101. [PubMed] [Google Scholar]
- Rodman J. S., Ingleby H. PLASMA CELL MASTITIS. Ann Surg. 1939 Jun;109(6):921–930. doi: 10.1097/00000658-193906000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELL S., WEIGLE W. O. Immunization to homologous milk in the rabbit. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:850–853. doi: 10.3181/00379727-107-26775. [DOI] [PubMed] [Google Scholar]
- Shinohara K. Histo-immunologische Untersuchungen in Mastopathie. Yonago Acta Med. 1968 Feb;12(1):1–21. [PubMed] [Google Scholar]
- TERPLAN K. L., WITEBSKY E., ROSE N. R., PAINE J. R., EGAN R. W. Experimental thyroiditis in rabbits, guinea pigs and dogs, following immunization with thyroid extracts of their own and of heterologous species. Am J Pathol. 1960 Feb;36:213–239. [PMC free article] [PubMed] [Google Scholar]
- Willoughby D. A., Coote E. The lymph-node permeability factor: a possible mediator of experimental allergic thyroiditis in the rat. J Pathol Bacteriol. 1966 Oct;92(2):281–289. doi: 10.1002/path.1700920204. [DOI] [PubMed] [Google Scholar]