Abstract
The effect was followed in the rat of 6 or 12 injections of isoprenaline (IPN), at a dose of 10 or 25 mg, and pilocarpine (PCP) at a dose of 10 mg.
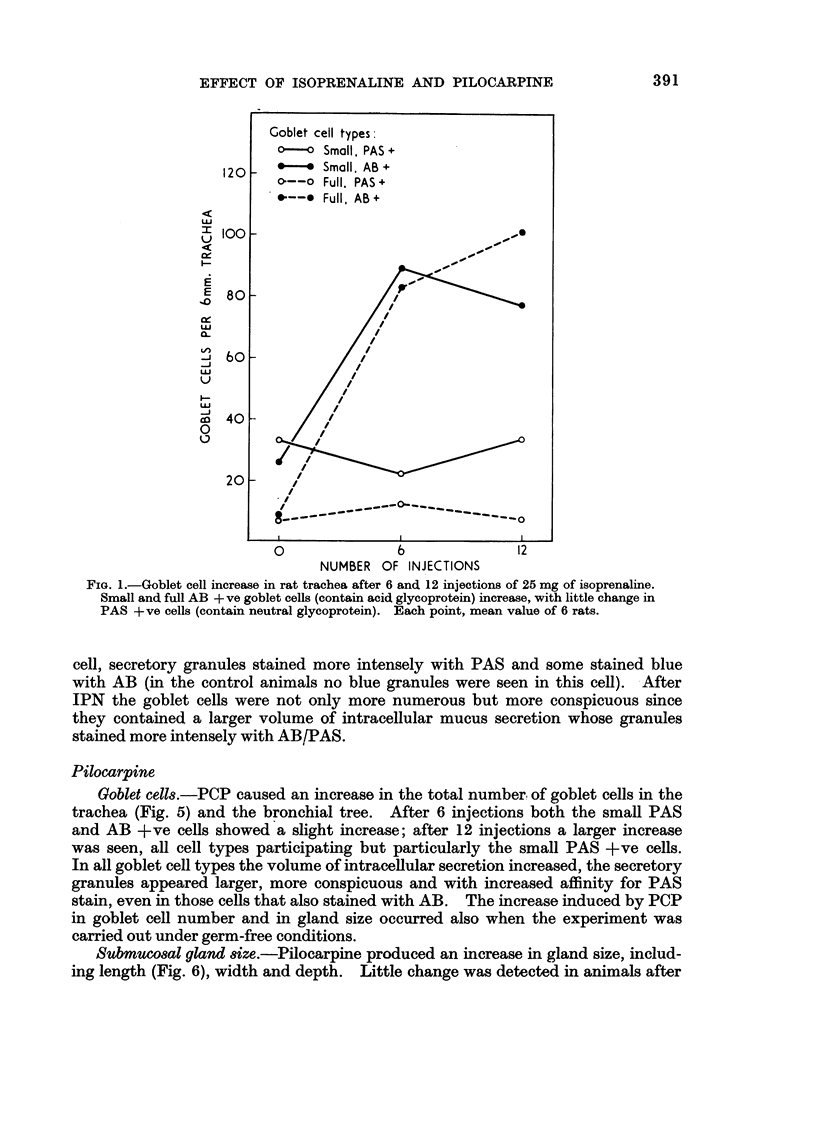
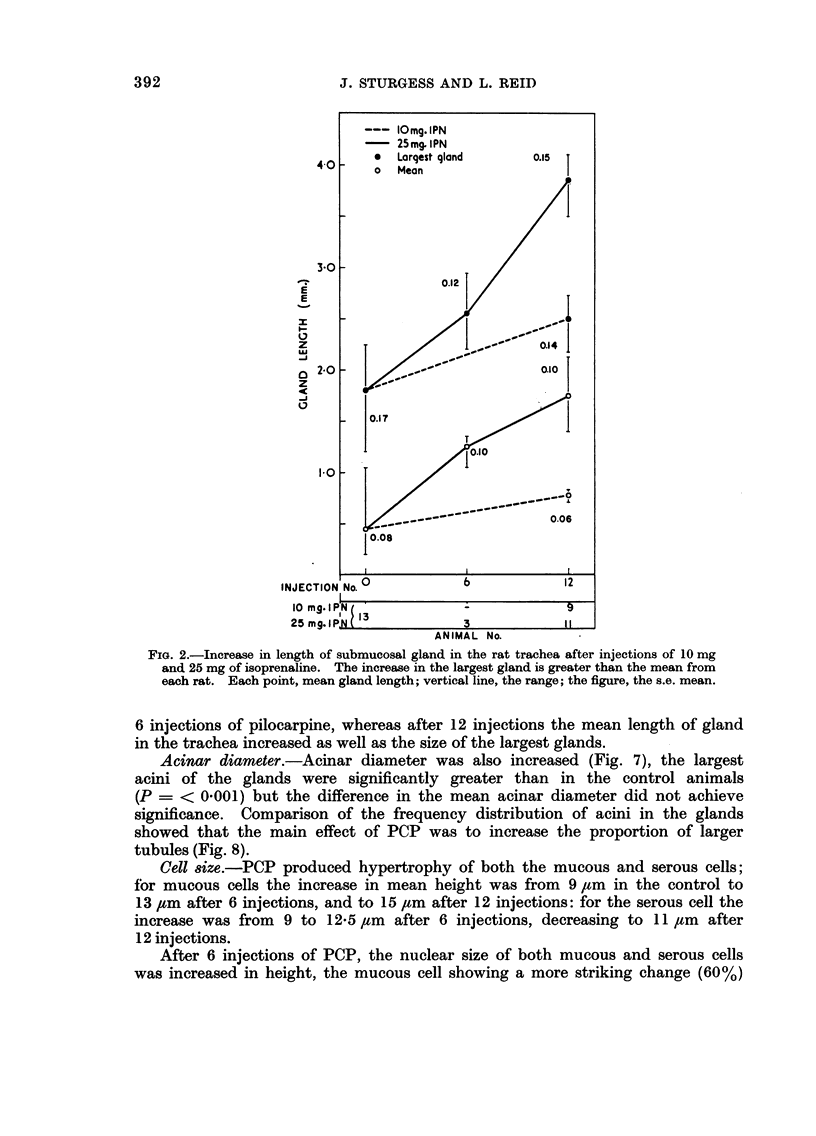
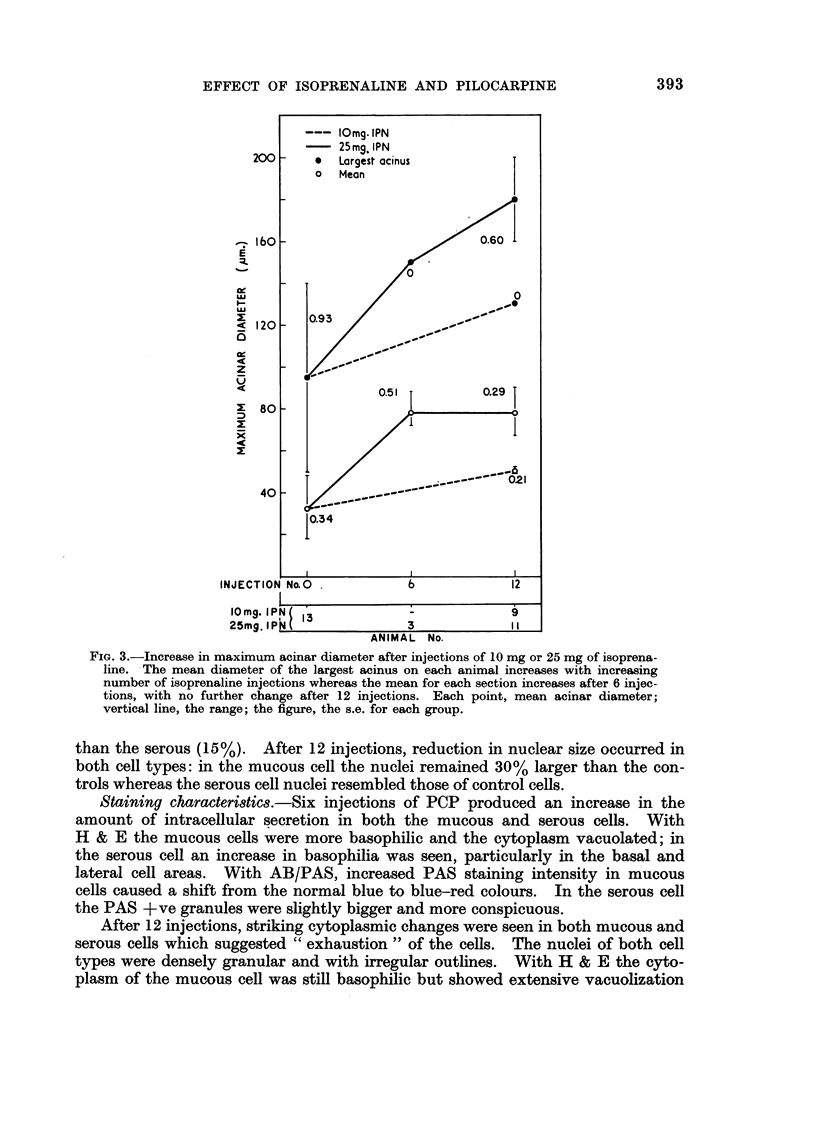
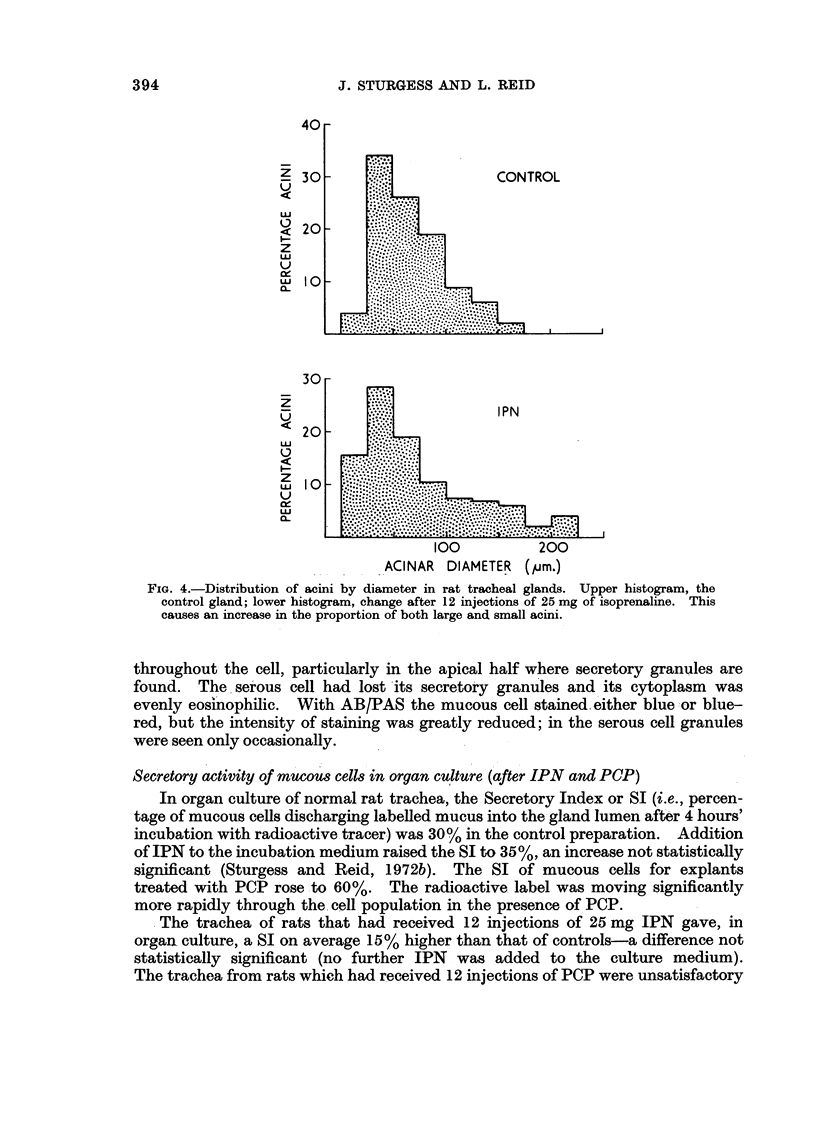
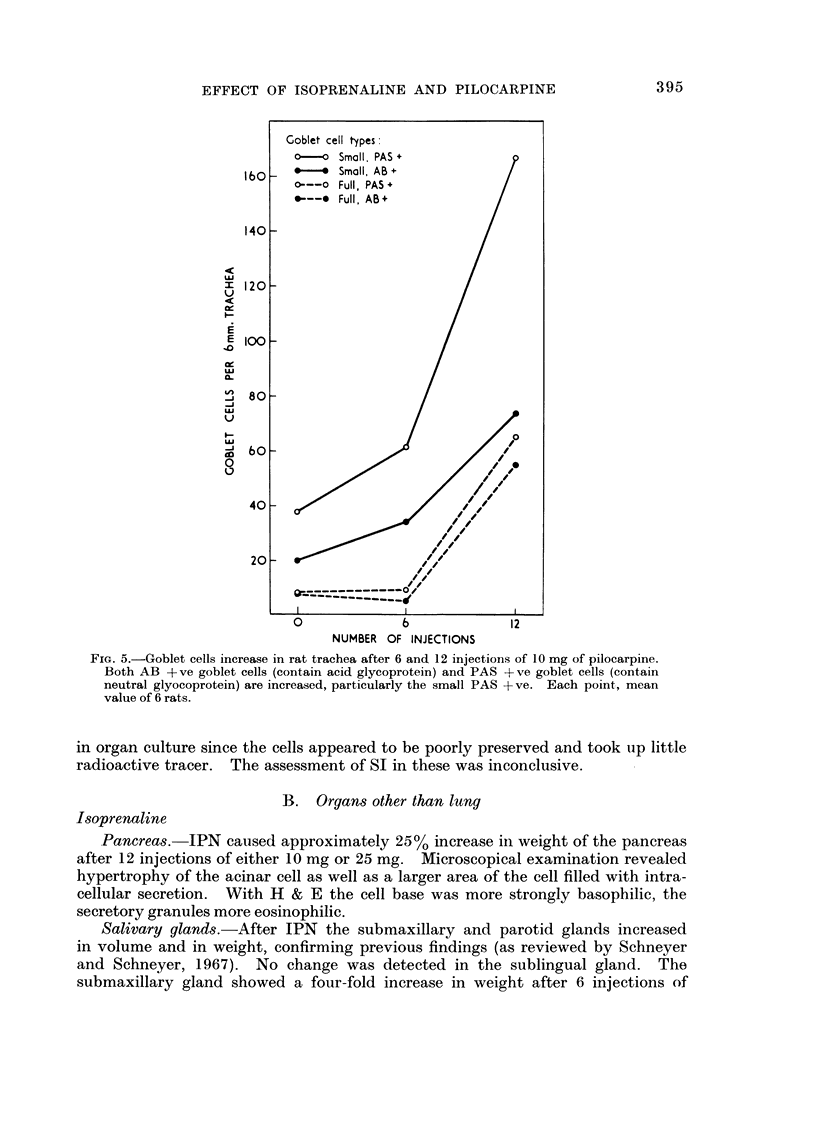
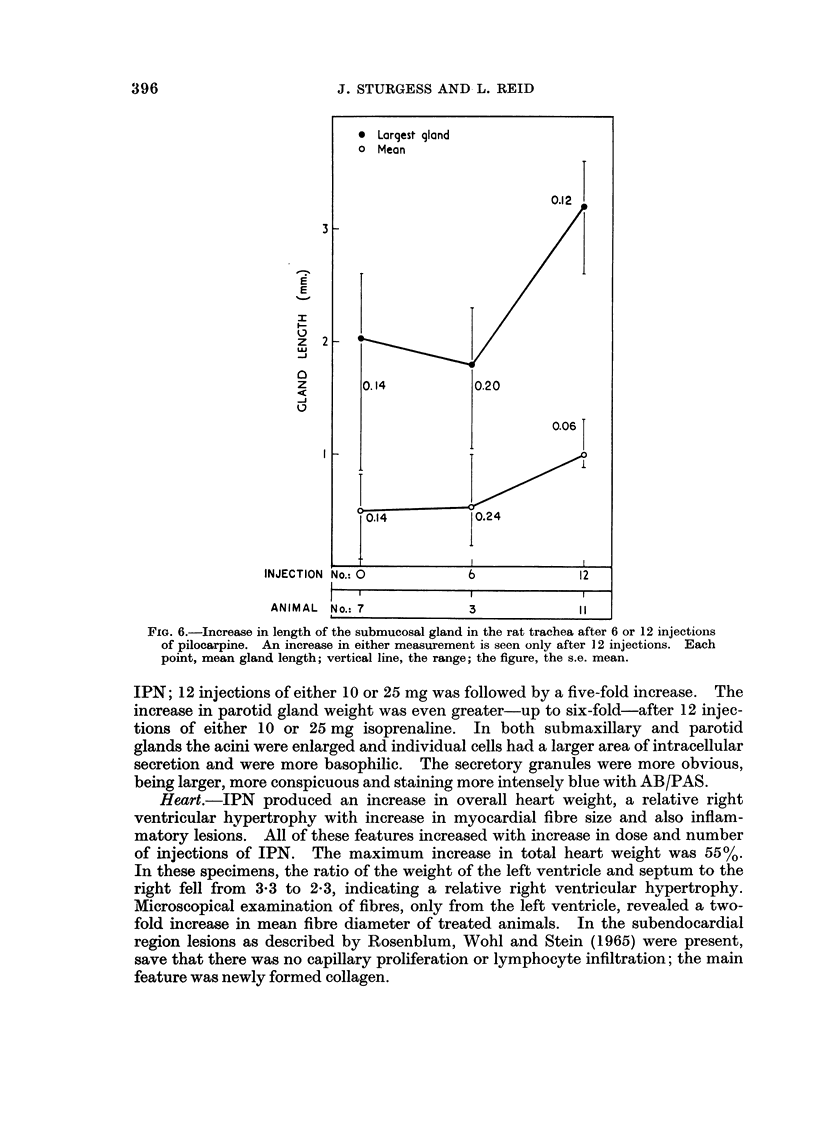
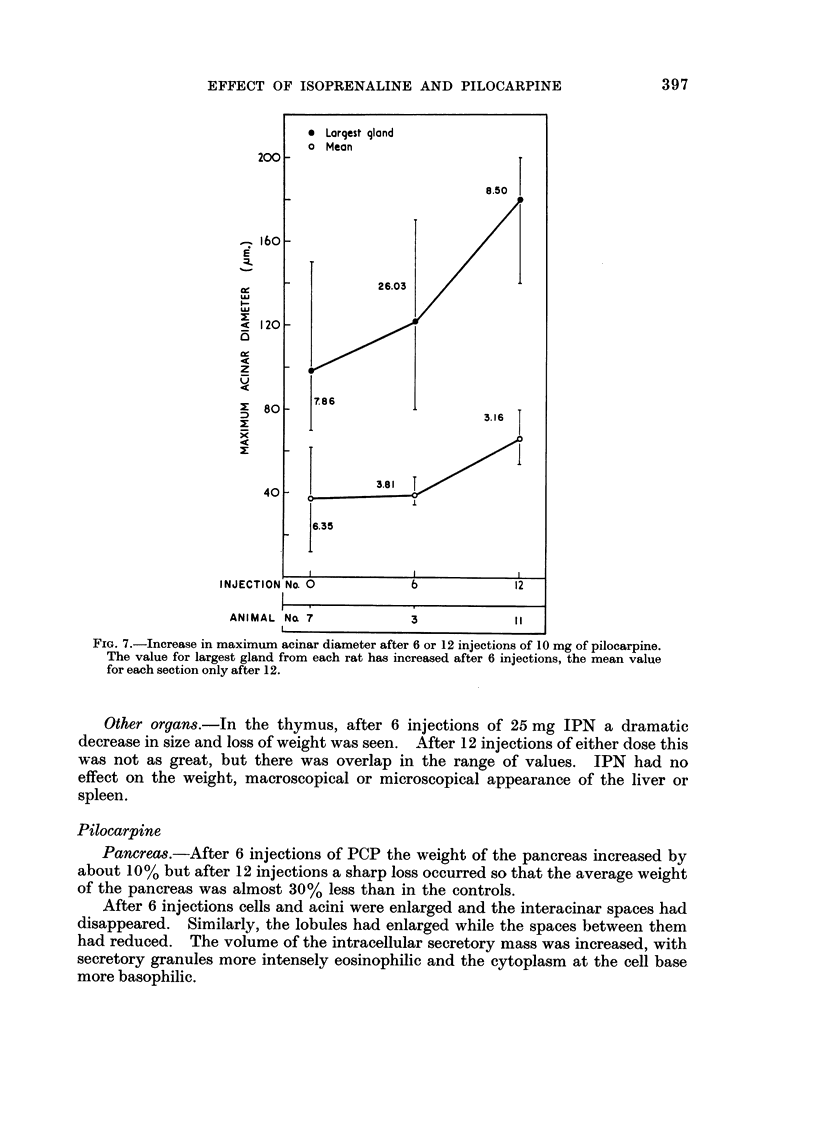
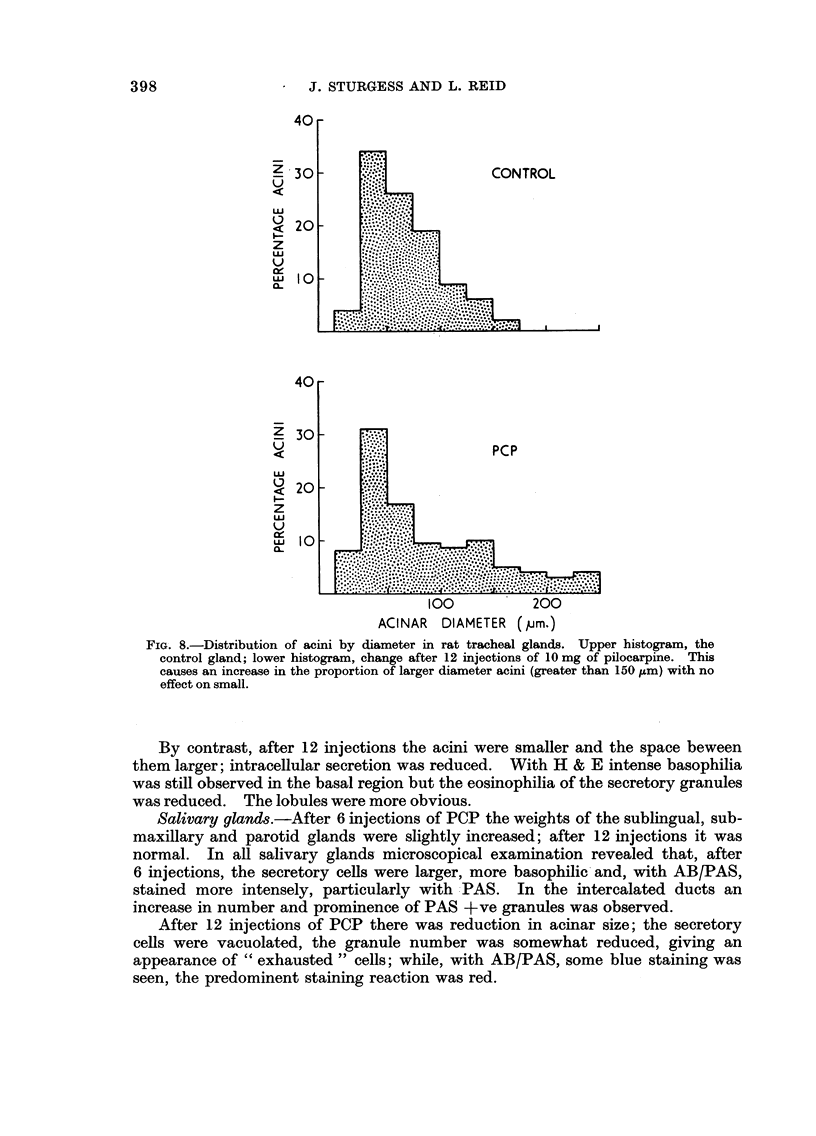
In some respects the effects are similar, in others strikingly dissimilar. IPN and PCP each increase bronchial submucosal gland size and the number of goblet cells previously thought not to be under nervous control. Isoprenaline increases goblet cells containing acid glycoprotein, the PCP all types: IPN increases small acini in the gland, PCP large ones. The IPN effect was apparent even under germ-free conditions. After 12 injections of PCP the secretory cells appeared “exhausted” and relatively empty of secretion.
A similar picture was seen in the pancreas and the salivary glands—hypertrophy after IPN or 6 injections of PCP, exhaustion after 12 of PCP. In the heart, IPN caused an increase in ventricular weight (the right more affected than the left), increase in fibre size and a minor degree of myocardial damage; PCP caused only dilatation. After 6 injections, both IPN and PCP reduced thymic weight; this had recovered after 12 injections. The effect of PCP seems to be at least in part directly on discharge; IPN seems to affect synthesis.
This is the first demonstration of goblet cell increase by drug effect. These changes are considered in relation to control of mucus secretion and to their relevance to cystic fibrosis.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD E. M., JARZYLO S. Chronic atropinization and fibrocystic disease of the pancreas. Can Med Assoc J. 1960 Apr 16;82:821–824. [PMC free article] [PubMed] [Google Scholar]
- Barbero G. J. Pathogenesis of cystic fibrosis: some current views. Ciba Found Study Group. 1968;32:2–16. [PubMed] [Google Scholar]
- CHAPPEL C. I., RONA G., BALAZS T., GAUDRY R. Comparison of cardiotoxic actions of certain sympathomimetic amines. Can J Biochem Physiol. 1959 Jan;37(1):35–42. [PubMed] [Google Scholar]
- De Haller R., Reid L. Adult chronic bronchitis. Morphology, histochemistry and vascularisation of the bronchial mucous glands. Med Thorac. 1965;22(6):549–567. [PubMed] [Google Scholar]
- FREEMAN G., HAYDON G. B. EMPHYSEMA AFTER LOW-LEVEL EXPOSURE TO NO2. Arch Environ Health. 1964 Jan;8:125–128. doi: 10.1080/00039896.1964.10663640. [DOI] [PubMed] [Google Scholar]
- FULTON R. M., HUTCHINSON E. C., JONES A. M. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952 Jul;14(3):413–420. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery P., Reid L. Intra-epithelial nerves in normal rat airways: a quantitative electron microscopic study. J Anat. 1973 Jan;114(Pt 1):35–45. [PMC free article] [PubMed] [Google Scholar]
- Jones R., Bolduc P., Reid L. Goblet cell glycoprotein and tracheal gland hypertrophy in rat airways: the effect of tobacco smoke with or without the anti-inflammatory agent phenylmethyloxadiazole. Br J Exp Pathol. 1973 Apr;54(2):229–239. [PMC free article] [PubMed] [Google Scholar]
- Jones R., Bolduc P., Reid L. Protection of rat bronchial epithelium against tobacco smoke. Br Med J. 1972 Apr 15;2(5806):142–144. doi: 10.1136/bmj.2.5806.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Reid L. The effect of pH on Alcian Blue staining of epithelial acid glycoproteins. I. Sialomucins and sulphomucins (singly or in simple combinations). Histochem J. 1973 Jan;5(1):9–18. doi: 10.1007/BF01012040. [DOI] [PubMed] [Google Scholar]
- Jones R., Reid L. The effect of pH on Alcian Blue staining of epithelial acid glycoproteins. II. Human bronchial submucosal gland. Histochem J. 1973 Jan;5(1):19–27. doi: 10.1007/BF01012041. [DOI] [PubMed] [Google Scholar]
- Lamb D., Reid L. Histochemical types of acidic glycoprotein produced by mucous cells of the tracheobronchial glands in man. J Pathol. 1969 Aug;98(4):213–229. doi: 10.1002/path.1710980402. [DOI] [PubMed] [Google Scholar]
- Lamb D., Reid L. Mitotic rates, goblet cell increase and histochemical changes in mucus in rat bronchial epithelium during exposure to sulphur dioxide. J Pathol Bacteriol. 1968 Jul;96(1):97–111. doi: 10.1002/path.1700960111. [DOI] [PubMed] [Google Scholar]
- Lamb D., Reid L. The tracheobronchial submucosal glands in cystic fibrosis: a qualitative and quantitative histochemical study. Br J Dis Chest. 1972 Oct;66(4):239–247. doi: 10.1016/0007-0971(72)90042-3. [DOI] [PubMed] [Google Scholar]
- REID L. AN EXPERIMENTAL STUDY OF HYPERSECRETION OF MUCUS IN THE BRONCHIAL TREE. Br J Exp Pathol. 1963 Aug;44:437–445. [PMC free article] [PubMed] [Google Scholar]
- REID L. Measurement of the bronchial mucous gland layer: a diagnostic yardstick in chronic bronchitis. Thorax. 1960 Jun;15:132–141. doi: 10.1136/thx.15.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS G. B. Fundamental defect in fibrocystic disease of the pancreas. Lancet. 1959 Nov 28;2(7109):964–965. doi: 10.1016/s0140-6736(59)91607-1. [DOI] [PubMed] [Google Scholar]
- ROSENBLUM I., WOHL A., STEIN A. A. STUDIES IN CARDIAC NECROSIS. I. PRODUCTION OF CARDIAC LESIONS WITH SYMPATHOMIMETIC AMINES. Toxicol Appl Pharmacol. 1965 Jan;7:1–8. doi: 10.1016/0041-008x(65)90067-0. [DOI] [PubMed] [Google Scholar]
- SCHNEYER C. A. Salivary gland changes after isoproterenol-induced enlargement. Am J Physiol. 1962 Aug;203:232–236. doi: 10.1152/ajplegacy.1962.203.2.232. [DOI] [PubMed] [Google Scholar]
- Sturgess J., Reid L. An organ culture study of the effect of drugs on the secretory activity of the human bronchial submucosal gland. Clin Sci. 1972 Oct;43(4):533–543. doi: 10.1042/cs0430533. [DOI] [PubMed] [Google Scholar]
- Sturgess J., Reid L. Secretory activity of the human bronchial mucous glands in vitro. Exp Mol Pathol. 1972 Jun;16(3):362–381. doi: 10.1016/0014-4800(72)90011-1. [DOI] [PubMed] [Google Scholar]
- WELLS H. The neural regulation of salivary gland growth. Ann N Y Acad Sci. 1963 Mar 30;106:654–667. doi: 10.1111/j.1749-6632.1963.tb16673.x. [DOI] [PubMed] [Google Scholar]


