Abstract
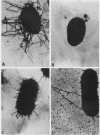
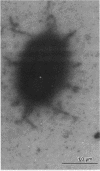
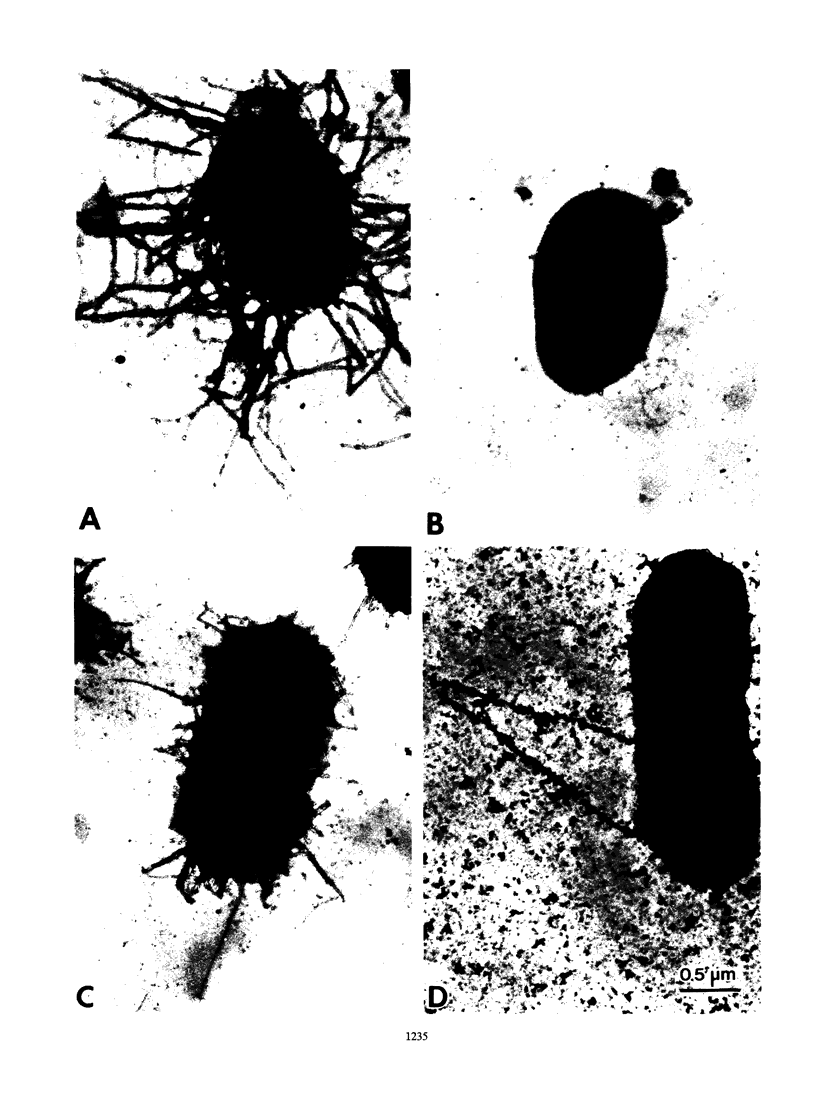
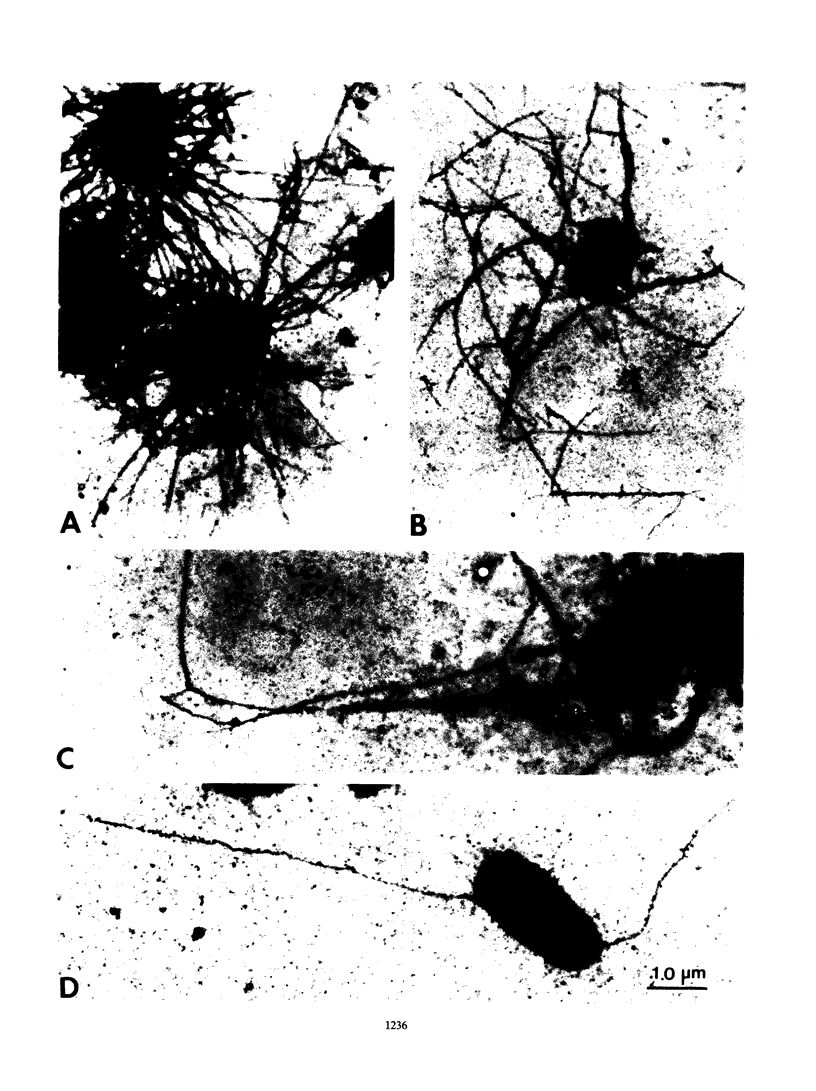
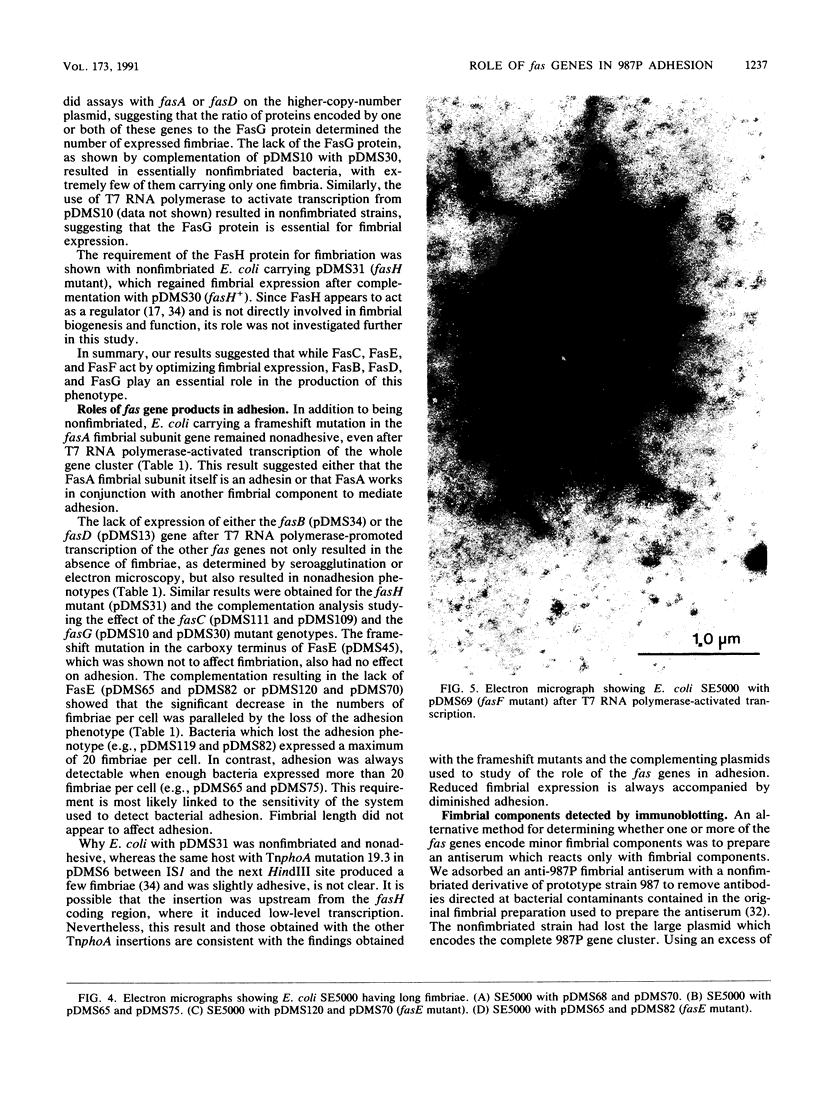
The 987P fimbrial gene cluster has recently been shown to contain eight genes (fasA to fasH) clustered on large plasmids of enterotoxigenic Escherichia coli and adjacent to a Tn1681-like transposon encoding the heat-stable enterotoxin STIa. Different genetic approaches were used to study the relationship between 987P fimbriation and adhesion. TnphoA mutagenesis, complementation assays, and T7 RNA polymerase-promoted gene expression indicated that all of the fas genes were involved in fimbrial expression and adhesion. In contrast to other fimbrial systems, the lack of expression of any single fas gene never resulted in the dissociation of fimbriation and adhesion, indicating that the adhesin is required for fimbrial expression and suggesting that FasA, the fimbrial structural subunit itself, is the adhesin. In addition, fimbrial length was shown to be modulated by the levels of expression of different fas genes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Goguen J. D., Sun D., Klemm P., Beachey E. H. Identification of two ancillary subunits of Escherichia coli type 1 fimbriae by using antibodies against synthetic oligopeptides of fim gene products. J Bacteriol. 1987 Dec;169(12):5530–5536. doi: 10.1128/jb.169.12.5530-5536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Mooi F. R. The fimbrial adhesins of Escherichia coli. Adv Microb Physiol. 1986;28:65–143. doi: 10.1016/s0065-2911(08)60237-4. [DOI] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. In vitro adhesion of piliated Escherichia coli to small intestinal villous epithelial cells from rabbits and the identification of a soluble 987P pilus receptor-containing fraction. Infect Immun. 1982 Jun;36(3):1192–1198. doi: 10.1128/iai.36.3.1192-1198.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. Location and distribution of a receptor for the 987P pilus of Escherichia coli in small intestines. Infect Immun. 1985 Feb;47(2):345–348. doi: 10.1128/iai.47.2.345-348.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. Purification and characterization of a receptor for the 987P pilus of Escherichia coli. Infect Immun. 1985 Jan;47(1):98–105. doi: 10.1128/iai.47.1.98-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Whipp S. C., Moon H. W. Age-specific colonization of porcine intestinal epithelium by 987P-piliated enterotoxigenic Escherichia coli. Infect Immun. 1989 Jan;57(1):82–87. doi: 10.1128/iai.57.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Richter P. Escherichia coli 987P pilus: purification and partial characterization. J Bacteriol. 1981 May;146(2):784–789. doi: 10.1128/jb.146.2.784-789.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., Simons B. H., de Graaf F. K. The role of lysine-132 and arginine-136 in the receptor-binding domain of the K99 fibrillar subunit. EMBO J. 1987 Jun;6(6):1805–1808. doi: 10.1002/j.1460-2075.1987.tb02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., Venema J., Leeven R., van Pelt-Heerschap H., de Graaf F. K. Inhibition of adhesive activity of K88 fibrillae by peptides derived from the K88 adhesin. J Bacteriol. 1987 Feb;169(2):735–741. doi: 10.1128/jb.169.2.735-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng S. T., Gardner J. F., Gumport R. I. Transcription termination by bacteriophage T7 RNA polymerase at rho-independent terminators. J Biol Chem. 1990 Mar 5;265(7):3823–3830. [PubMed] [Google Scholar]
- Klaasen P., Woodward M. J., van Zijderveld F. G., de Graaf F. K. The 987P gene cluster in enterotoxigenic Escherichia coli contains an STpa transposon that activates 987P expression. Infect Immun. 1990 Mar;58(3):801–807. doi: 10.1128/iai.58.3.801-807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F. P., Lund B., Normark S. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 1984 May;3(5):1167–1173. doi: 10.1002/j.1460-2075.1984.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister W. T., Morris C., Rosenberg A. H., Studier F. W. Utilization of bacteriophage T7 late promoters in recombinant plasmids during infection. J Mol Biol. 1981 Dec 15;153(3):527–544. doi: 10.1016/0022-2836(81)90406-x. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., Claassen I., Bakker D., Kuipers H., de Graaf F. K. Regulation and structure of an Escherichia coli gene coding for an outer membrane protein involved in export of K88ab fimbrial subunits. Nucleic Acids Res. 1986 Mar 25;14(6):2443–2457. doi: 10.1093/nar/14.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine intestine by enterotoxigenic Escherichia coli: selection of piliated forms in vivo, adhesion of piliated forms to epithelial cells in vitro, and incidence of a pilus antigen among porcine enteropathogenic E. coli. Infect Immun. 1977 Apr;16(1):344–352. doi: 10.1128/iai.16.1.344-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Båga M., Tennent J. M., Normark S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol. 1987 Sep;1(2):169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Courtney H. S., Schifferli D. M., Beachey E. H. Enzyme-linked immunosorbent assay for adherence of bacteria to animal cells. J Clin Microbiol. 1986 Oct;24(4):512–516. doi: 10.1128/jcm.24.4.512-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., de Graaf M., de Boer L., Bakker D., Vader C. E., Mooi F. R., de Graaf F. K. Detection and identification of FaeC as a minor component of K88 fibrillae of Escherichia coli. Mol Microbiol. 1989 May;3(5):645–652. doi: 10.1111/j.1365-2958.1989.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Riegman N., Kusters R., Van Veggel H., Bergmans H., Van Bergen en Henegouwen P., Hacker J., Van Die I. F1C fimbriae of a uropathogenic Escherichia coli strain: genetic and functional organization of the foc gene cluster and identification of minor subunits. J Bacteriol. 1990 Feb;172(2):1114–1120. doi: 10.1128/jb.172.2.1114-1120.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegman N., van Die I., Leunissen J., Hoekstra W., Bergmans H. Biogenesis of F7(1) and F7(2) fimbriae of uropathogenic Escherichia coli: influence of the FsoF and FstFG proteins and localization of the Fso/FstE protein. Mol Microbiol. 1988 Jan;2(1):73–80. [PubMed] [Google Scholar]
- Schifferli D. M., Abraham S. N., Beachey E. H. Use of monoclonal antibodies to probe subunit- and polymer-specific epitopes of 987P fimbriae of Escherichia coli. Infect Immun. 1987 Apr;55(4):923–930. doi: 10.1128/iai.55.4.923-930.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli D. M., Beachey E. H., Taylor R. K. The 987P fimbrial gene cluster of enterotoxigenic Escherichia coli is plasmid encoded. Infect Immun. 1990 Jan;58(1):149–156. doi: 10.1128/iai.58.1.149-156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Hoschützky H., Morschhäuser J., Lottspeich F., Jann K., Hacker J. Analysis of genes coding for the sialic acid-binding adhesin and two other minor fimbrial subunits of the S-fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1989 Dec;3(12):1735–1744. doi: 10.1111/j.1365-2958.1989.tb00159.x. [DOI] [PubMed] [Google Scholar]
- So M., Atchison R., Falkow S., Moseley S., McCarthy B. J. A study of the dissemination of Tn1681: a bacterial transposon encoding a heat-stable toxin among enterotoxigenic Escherichia coli isolates. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):53–58. doi: 10.1101/sqb.1981.045.01.010. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Klaasen P. Organization and expression of genes involved in the biosynthesis of 987P fimbriae. Mol Gen Genet. 1986 Jul;204(1):75–81. doi: 10.1007/BF00330190. [DOI] [PubMed] [Google Scholar]