Abstract
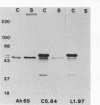
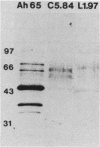
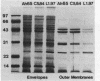
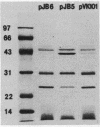
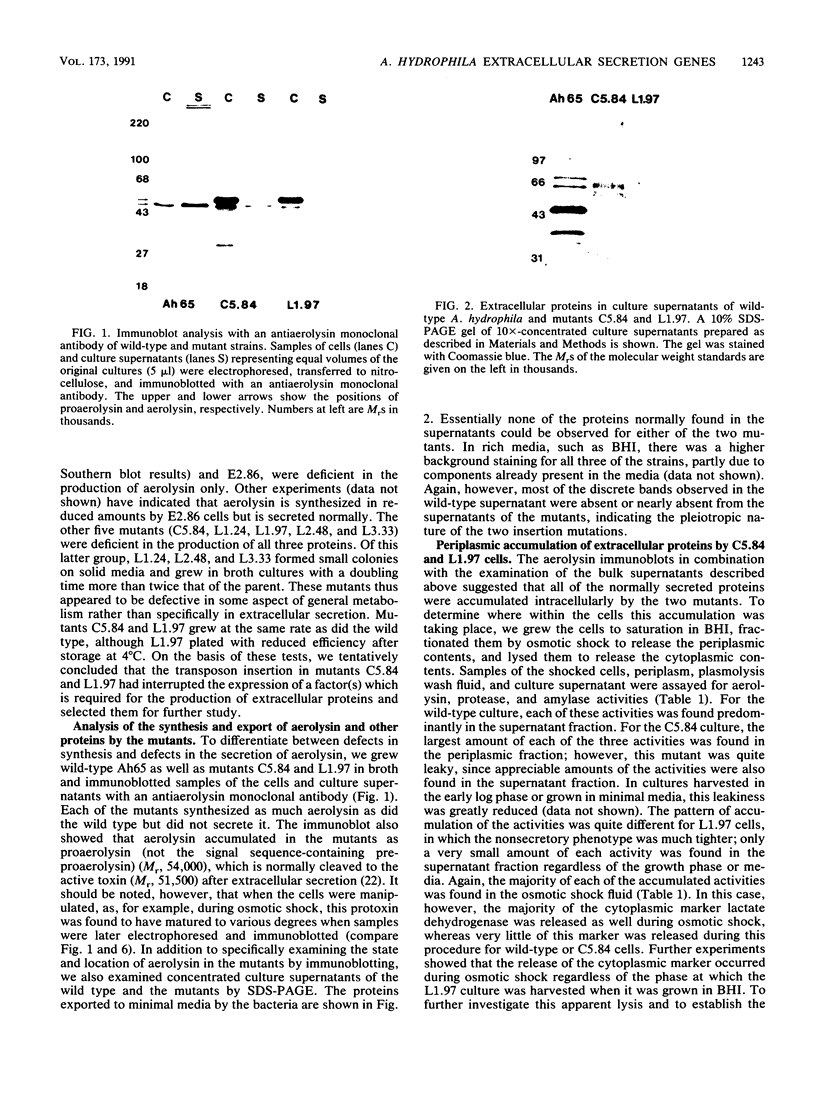
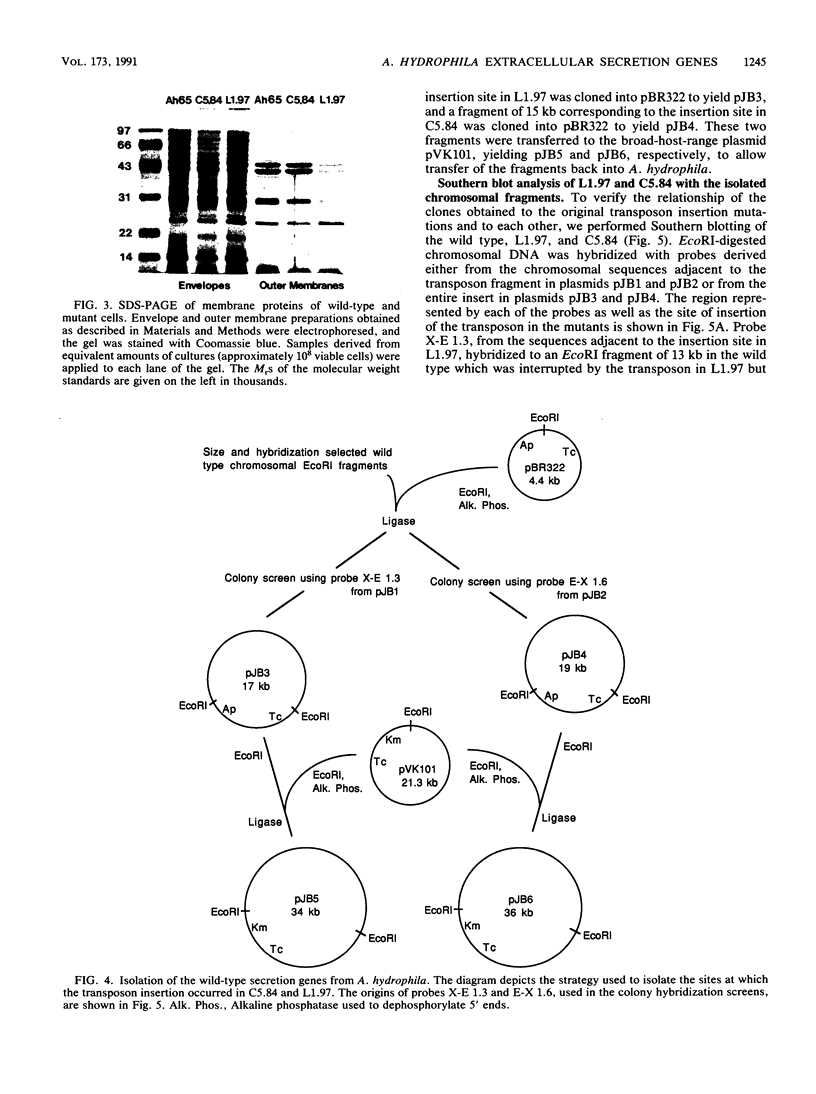
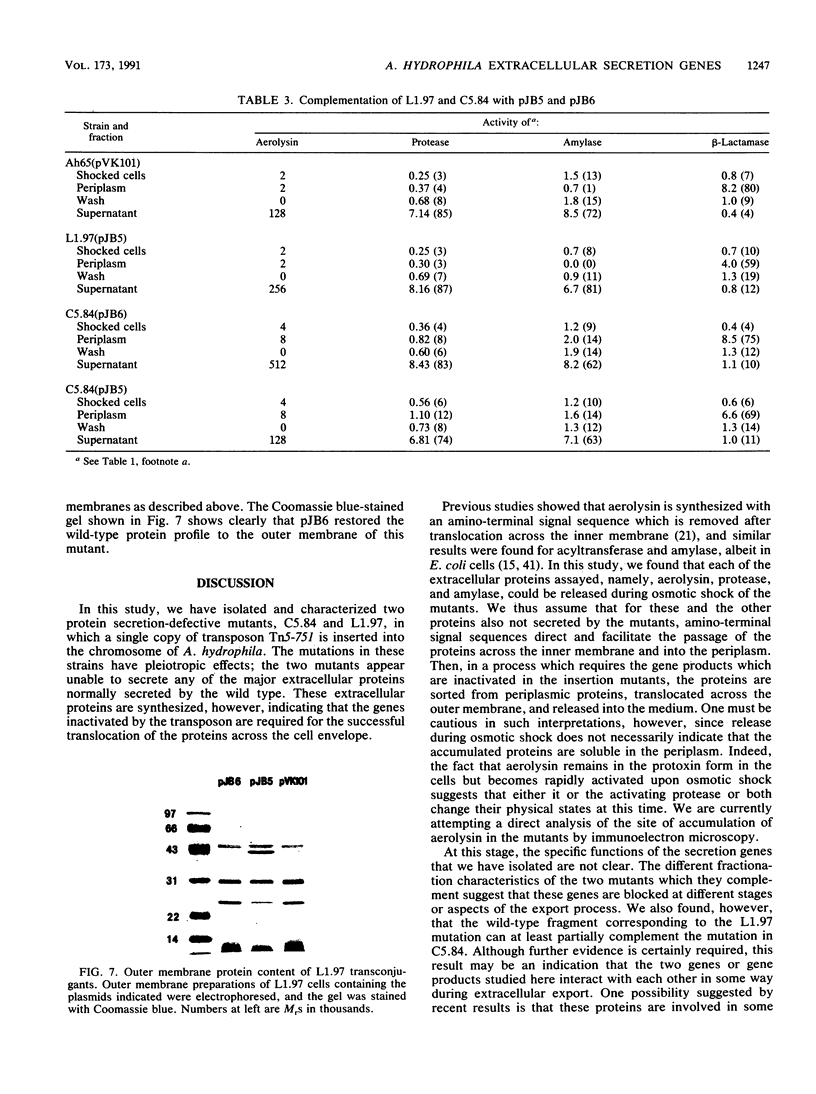
Transposon mutagenesis was used to isolate mutants of Aeromonas hydrophila which were deficient in the production of extracellular proteins. The culture supernatants of two of the mutants were essentially devoid of the proteins normally secreted by the parent strain, despite their continued synthesis. Western immunoblot analysis of one of these proteins indicated that normal signal sequence processing occurred but that normal zymogen activation did not, and cell fractionation experiments indicated that both mutants accumulated the three different extracellular proteins assayed in a position external to the cytoplasmic membrane, presumably in the periplasm. The two mutants differed, however, in that one was lysed during the osmotic shock procedures and also contained severely reduced amounts of two of the major protein components of the outer membrane. The wild-type chromosomal regions into which the transposon had been inserted in the two mutants were cloned. In each case, transconjugants of the mutants containing the corresponding cloned fragment were complemented for the defects in secretion, and one of the mutants was complemented by the heterologous clone as well, suggesting the possibility of an interaction between these two genes or gene products. These results indicate that two separate functions which are required for extracellular secretion were interrupted in the insertion mutants and that one of these is also critically important in the biogenesis of the outer membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bally M., Wretlind B., Lazdunski A. Protein secretion in Pseudomonas aeruginosa: molecular cloning and characterization of the xcp-1 gene. J Bacteriol. 1989 Aug;171(8):4342–4348. doi: 10.1128/jb.171.8.4342-4348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chakraborty T., Huhle B., Bergbauer H., Goebel W. Cloning, expression, and mapping of the Aeromonas hydrophila aerolysin gene determinant in Escherichia coli K-12. J Bacteriol. 1986 Jul;167(1):368–374. doi: 10.1128/jb.167.1.368-374.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Montenegro M. A., Sanyal S. C., Helmuth R., Bulling E., Timmis K. N. Cloning of enterotoxin gene from Aeromonas hydrophila provides conclusive evidence of production of a cytotonic enterotoxin. Infect Immun. 1984 Nov;46(2):435–441. doi: 10.1128/iai.46.2.435-441.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavira R., Jr, Burnett T. J., Hageman J. H. Assaying proteinases with azocoll. Anal Biochem. 1984 Feb;136(2):446–450. doi: 10.1016/0003-2697(84)90242-2. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., MacIntyre S., Buckley J. T., Hancock R. E. Purification and reconstitution in lipid bilayer membranes of an outer membrane, pore-forming protein of Aeromonas salmonicida. J Bacteriol. 1983 Dec;156(3):1006–1011. doi: 10.1128/jb.156.3.1006-1011.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A., Bally M., Murgier M., Wretlind B., Lazdunski A. Cloning of xcp genes located at the 55 min region of the chromosome and involved in protein secretion in Pseudomonas aeruginosa. Mol Microbiol. 1989 Feb;3(2):261–265. doi: 10.1111/j.1365-2958.1989.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Gobius K. S., Pemberton J. M. Molecular cloning, characterization, and nucleotide sequence of an extracellular amylase gene from Aeromonas hydrophila. J Bacteriol. 1988 Mar;170(3):1325–1332. doi: 10.1128/jb.170.3.1325-1332.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo J., Murgier M., Filloux A., Lazdunski A. Cloning of the Pseudomonas aeruginosa alkaline protease gene and secretion of the protease into the medium by Escherichia coli. J Bacteriol. 1990 Feb;172(2):942–948. doi: 10.1128/jb.172.2.942-948.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Lupo M. Glutamate transport in wild-type and mutant strains of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1288–1295. doi: 10.1128/jb.90.5.1288-1295.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Activation of the hole-forming toxin aerolysin by extracellular processing. J Bacteriol. 1985 Jul;163(1):336–340. doi: 10.1128/jb.163.1.336-340.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Intracellular accumulation of extracellular proteins by pleiotropic export mutants of Aeromonas hydrophila. J Bacteriol. 1983 Apr;154(1):413–418. doi: 10.1128/jb.154.1.413-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Molecular cloning and expression in Escherichia coli of the structural gene for the hemolytic toxin aerolysin from Aeromonas hydrophila. Mol Gen Genet. 1986 Aug;204(2):289–295. doi: 10.1007/BF00425512. [DOI] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Protein export by a gram-negative bacterium: production of aerolysin by Aeromonas hydrophila. J Bacteriol. 1985 Mar;161(3):1118–1124. doi: 10.1128/jb.161.3.1118-1124.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichige A., Oishi K., Mizushima S. Isolation and characterization of mutants of a marine Vibrio strain that are defective in production of extracellular proteins. J Bacteriol. 1988 Aug;170(8):3537–3542. doi: 10.1128/jb.170.8.3537-3542.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Senior B., Koronakis E., Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Koronakis E., Hughes C. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 1989 Feb;8(2):595–605. doi: 10.1002/j.1460-2075.1989.tb03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo R. Y., Strathdee C. A., Shewen P. E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987 Sep;55(9):1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S., Tai P. C. Characterization of the phospholipase C gene of Pseudomonas aeruginosa cloned in Escherichia coli. Gene. 1983 Apr;22(1):95–101. doi: 10.1016/0378-1119(83)90068-9. [DOI] [PubMed] [Google Scholar]
- Létoffé S., Delepelaire P., Wandersman C. Protease secretion by Erwinia chrysanthemi: the specific secretion functions are analogous to those of Escherichia coli alpha-haemolysin. EMBO J. 1990 May;9(5):1375–1382. doi: 10.1002/j.1460-2075.1990.tb08252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Secretion of haemolysin by Escherichia coli. Curr Top Microbiol Immunol. 1986;125:159–181. doi: 10.1007/978-3-642-71251-7_10. [DOI] [PubMed] [Google Scholar]
- Rella M., Mercenier A., Haas D. Transposon insertion mutagenesis of Pseudomonas aeruginosa with a Tn5 derivative: application to physical mapping of the arc gene cluster. Gene. 1985;33(3):293–303. doi: 10.1016/0378-1119(85)90237-9. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Wilding P., Haverback B. J. A new method for the determination of alpha-amylase. Experientia. 1967 Oct 15;23(10):805–805. doi: 10.1007/BF02146851. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stambaugh R., Post D. Substrate and product inhibition of rabbit muscle lactic dehydrogenase heart (H4) and muscle (M4) isozymes. J Biol Chem. 1966 Apr 10;241(7):1462–1467. [PubMed] [Google Scholar]
- Thornton J., Howard S. P., Buckley J. T. Molecular cloning of a phospholipid-cholesterol acyltransferase from Aeromonas hydrophila. Sequence homologies with lecithin-cholesterol acyltransferase and other lipases. Biochim Biophys Acta. 1988 Mar 25;959(2):153–159. doi: 10.1016/0005-2760(88)90026-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. R., Buckley J. T. Proton motive force involved in protein transport across the outer membrane of Aeromonas salmonicida. Science. 1989 Nov 3;246(4930):654–656. doi: 10.1126/science.2814486. [DOI] [PubMed] [Google Scholar]
- Wong K. R., Green M. J., Buckley J. T. Extracellular secretion of cloned aerolysin and phospholipase by Aeromonas salmonicida. J Bacteriol. 1989 May;171(5):2523–2527. doi: 10.1128/jb.171.5.2523-2527.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. R., McLean D. M., Buckley J. T. Cloned aerolysin of Aeromonas hydrophila is exported by a wild-type marine Vibrio strain but remains periplasmic in pleiotropic export mutants. J Bacteriol. 1990 Jan;172(1):372–376. doi: 10.1128/jb.172.1.372-376.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato I., Anraku Y., Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem. 1975 Apr;77(4):705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]
- d'Enfert C., Reyss I., Wandersman C., Pugsley A. P. Protein secretion by gram-negative bacteria. Characterization of two membrane proteins required for pullulanase secretion by Escherichia coli K-12. J Biol Chem. 1989 Oct 15;264(29):17462–17468. [PubMed] [Google Scholar]
- d'Enfert C., Ryter A., Pugsley A. P. Cloning and expression in Escherichia coli of the Klebsiella pneumoniae genes for production, surface localization and secretion of the lipoprotein pullulanase. EMBO J. 1987 Nov;6(11):3531–3538. doi: 10.1002/j.1460-2075.1987.tb02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]