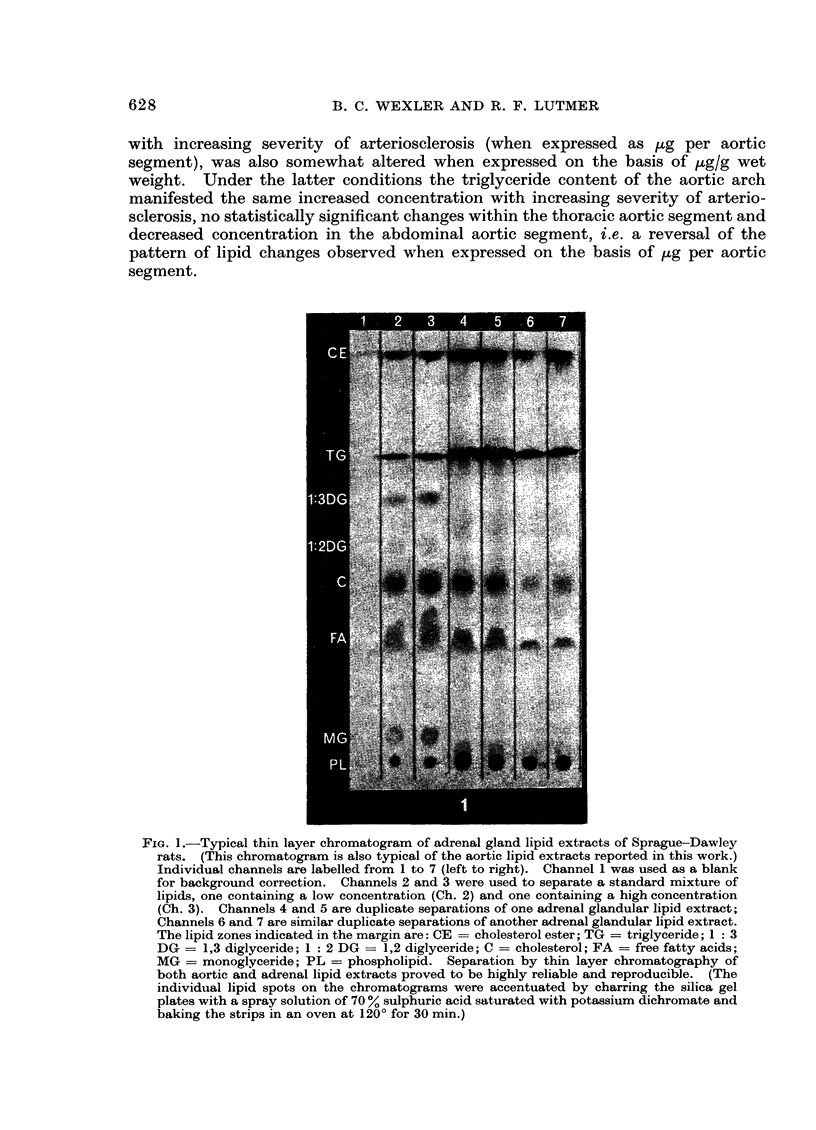
Abstract
Repeatedly bred male and female rats develop arteriosclerosis spontaneously. Despite hyperlipidaemia, fatty liver and obesity the arterial lesions contain minuscule quantities of lipid. Because histopathological lipid techniques may not detect bound or “masked” lipid, we used thin layer chromatography, which affords extra sensitivity in detecting small quantities of lipids. A survey was made of the lipids in female breeder aortae on the basis of the severity of their grossly-visible arteriosclerosis. The aortae were divided into arch, thoracic and abdominal aortic segments to further delineate any lipid changes, according to the anatomical pathogenesis of the arterial disease. Adrenal lipid changes were also analysed by the TLC procedure, comparing adrenal glands of non-arteriosclerotic animals with those having severe, grossly visible arteriosclerosis.
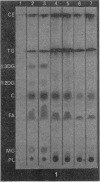
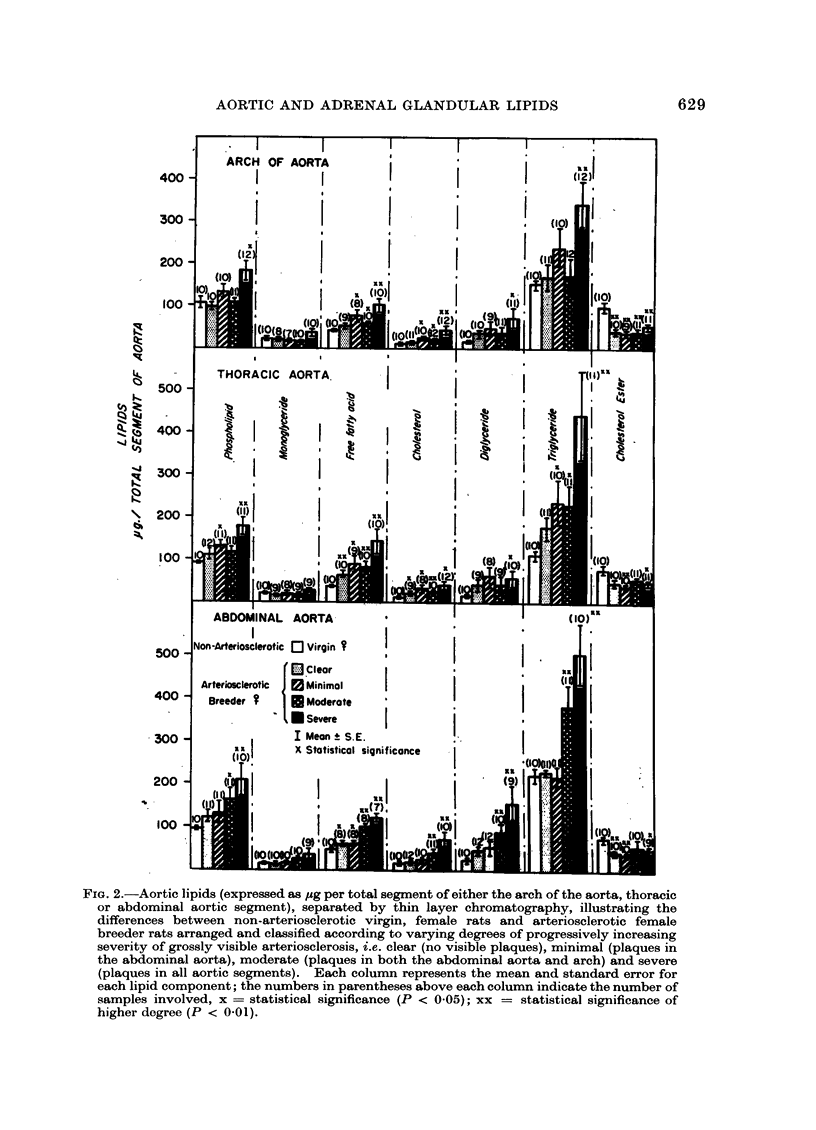
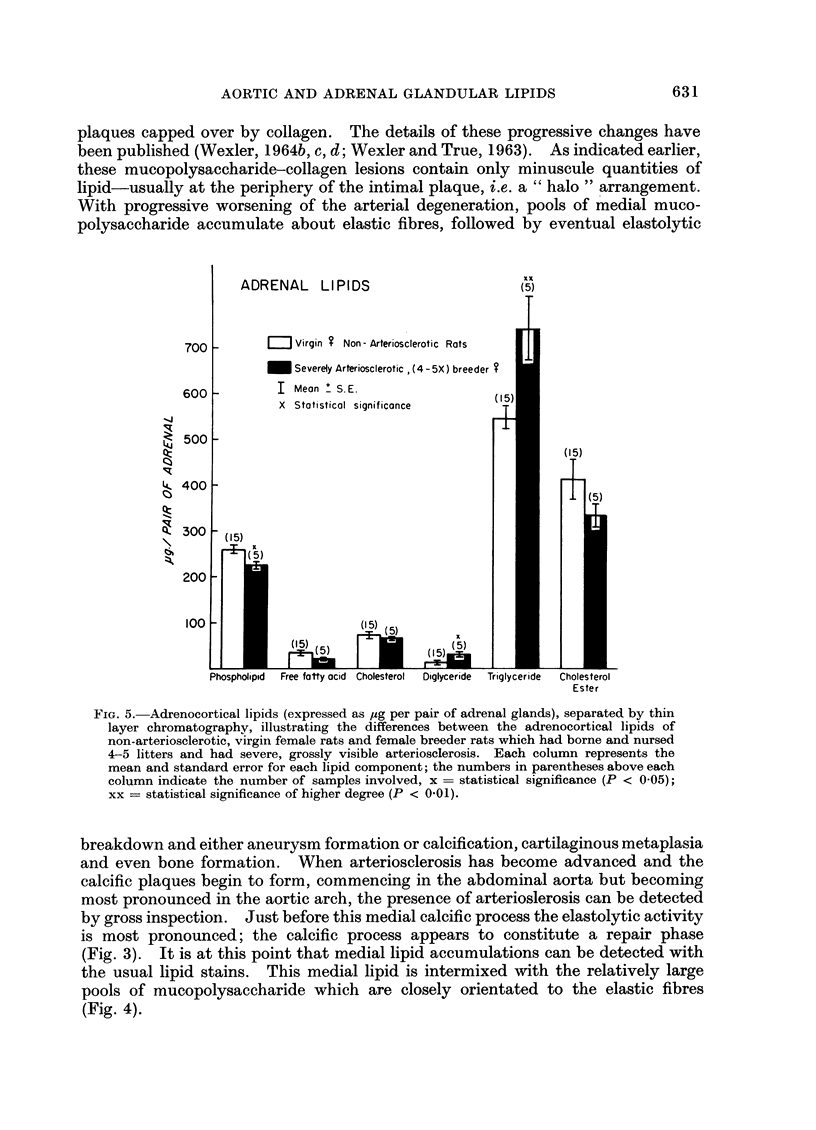
The TLC method demonstrated that small quantities of lipid accumulate in the aortae of repeatedly bred rats, first in the abdominal aortic segment, increasing in concentration with progressive severity of arteriosclerosis and spreading from abdominal aorta to arch and thoracic aorta. Specifically, phospholipids, free fatty acids, cholesterol, di- and triglycerides are increased whereas cholesterol ester is decreased as arteriosclerosis becomes more advanced. Adrenocortical di- and triglycerides were found to be greatly increased in those animals having severe, grossly visible arteriosclerosis. However, total cholesterol was reduced. These adrenal lipid changes are construed to be a reflection of the eventual impairment of the steroidogenic capacity of arteriosclerotic breeder rats. Our conclusions are that the stimulation of the hypothalamic-pituitary-adrenal-gonadal axis occasioned by repeated breeding invokes first a condition of hyperadrenocorticism, followed by adrenal “exhaustion”. The excess adrenal (and gonadal) steroids condition these animals towards hyperlipidaemia, fatty metamorphosis of the liver and obesity. However, these endogenous lipid changes do not contribute directly to the pathogenesis of the arterial lesions.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGELICO R., CAVINA G., D ANTONA A., GIOCOLI G. FRACTIONATION AND DETERMINATION OF THE LIPID AND STEROID CONSTITUENTS OF THE ADRENAL GLANDS OF RATS BY MEANS OF THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1965 Apr;18:57–68. doi: 10.1016/s0021-9673(01)80320-4. [DOI] [PubMed] [Google Scholar]
- Alfin-Slater R. B., Aftergood L. Lipids and the pill. Lipids. 1971 Oct;6(10):693–705. doi: 10.1007/BF02531293. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Judd J. T., Wexler B. C. The role of lactation and weaning in the pathogenesis of arteriosclerosis in female breeder rats. J Atheroscler Res. 1969 Sep-Oct;10(2):153–172. doi: 10.1016/s0368-1319(69)80004-9. [DOI] [PubMed] [Google Scholar]
- Kramsch D. M., Franzblau C., Hollander W. The protein and lipid composition of arterial elastin and its relationship to lipid accumulation in the atherosclerotic plaque. J Clin Invest. 1971 Aug;50(8):1666–1677. doi: 10.1172/JCI106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likar I. N., Likar L. J., Robinson R. W. Bovine arterial disease. II. Lipid distribution and pattern in abdominal aorta with and without naturally occurring gross lesions. Arch Pathol. 1966 Dec;82(6):561–565. [PubMed] [Google Scholar]
- Likar I. N., Robinson R. W. Bovine arterial disease. I. Localization of lipids in the abdominal aorta in relation to bovine atherosclerosis. Arch Pathol. 1966 Dec;82(6):555–560. [PubMed] [Google Scholar]
- MOSINGER F. PHOTOMETRIC ADAPTATION OF DOLE'S MICRODETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jan;6:157–159. [PubMed] [Google Scholar]
- Macho L., Saffran M. Metabolism of fatty acids in the rat adrenal gland. Endocrinology. 1967 Aug;81(2):179–185. doi: 10.1210/endo-81-2-179. [DOI] [PubMed] [Google Scholar]
- Rudman D., Garcia L. A. Effect of adrenocorticotropin on the concentration of triglyceride in the adrenal gland of the hypophysectomized rat. Endocrinology. 1966 May;78(5):1087–1088. doi: 10.1210/endo-78-5-1087. [DOI] [PubMed] [Google Scholar]
- Saffran J., Saffran M., Salhanick H. A. Clofibrate inhibits lipid synthesis and ACTH-stimulated steroidogenesis by rat adrenal tissue. Endocrinology. 1970 Mar;86(3):652–655. doi: 10.1210/endo-86-3-652. [DOI] [PubMed] [Google Scholar]
- Tucker M. J. The effect of clofibrate on spontaneous arteriosclerosis in rats. Atherosclerosis. 1971 Mar-Apr;13(2):255–265. doi: 10.1016/0021-9150(71)90028-1. [DOI] [PubMed] [Google Scholar]
- WEXLER B. C. CORRELATION OF ADRENOCORTICAL HISTOPATHOLOGY WITH ARTERIOSCLEROSIS IN BREEDER RATS. Acta Endocrinol (Copenh) 1964 Aug;46:613–631. doi: 10.1530/acta.0.0460613. [DOI] [PubMed] [Google Scholar]
- WEXLER B. C. SPONTANEOUS ARTERIOSCLEROSIS OF THE MESENTERIC, RENAL, AND PERIPHERAL ARTERIES OF REPEATEDLY BRED RATS. Circ Res. 1964 Dec;15:485–496. doi: 10.1161/01.res.15.6.485. [DOI] [PubMed] [Google Scholar]
- WEXLER B. C. SPONTANEOUS CORONARY ARTERIOSCLEROSIS IN REPEATEDLY BRED MALE AND FEMALE RATS. Circ Res. 1964 Jan;14:32–43. doi: 10.1161/01.res.14.1.32. [DOI] [PubMed] [Google Scholar]
- WEXLER B. C., TRUE C. W. Carotid and cerebral arteriosclerosis in the rat. Circ Res. 1963 Jun;12:659–666. doi: 10.1161/01.res.12.6.659. [DOI] [PubMed] [Google Scholar]
- Wexler B. C. Histopathologic responses to severe alloxan diabetes in arteriosclerotic and nonarteriosclerotic rats. Diabetes. 1970 May;19(5):324–336. doi: 10.2337/diab.19.5.324. [DOI] [PubMed] [Google Scholar]
- Wexler B. C., Saroff J. Divergent responses of arteriosclerotic and non-arteriosclerotic rats to a catabolic dose of cortisone. Acta Endocrinol (Copenh) 1969 Jul;61(3):509–524. doi: 10.1530/acta.0.0610509. [DOI] [PubMed] [Google Scholar]
- Wexler B. C., Saroff J., Judd J. Variations in response to severe alloxan diabetes in arteriosclerotic and nonarteriosclerotic rats. Diabetes. 1970 May;19(5):311–323. doi: 10.2337/diab.19.5.311. [DOI] [PubMed] [Google Scholar]
- Wuthier R. E., Irving J. T. A study of the lipids of the rat aorta during induced calcification. Proc Soc Exp Biol Med. 1969 Jan;130(1):156–162. doi: 10.3181/00379727-130-33511. [DOI] [PubMed] [Google Scholar]