Abstract
Lewis rats show greater anticipatory contrast effects than Fischer 344 rats. Specifically, relative to Fischer rats, Lewis rats exhibit greater avoidance of a saccharin cue when it predicts the future availability of a preferred sucrose reward [Grigson, P.S., Freet, C.S. The suppressive effects of sucrose and cocaine, but not lithium chloride, are greater in Lewis than in Fischer rats: evidence for the reward comparison hypothesis. Behav Neurosci 2000;114:353–363.]. Experiment 1 was designed to determine whether Lewis rats also would demonstrate greater contrast in another paradigm, successive negative contrast (SNC). The results demonstrated a tendency for greater SNC in Lewis rats and then slower recovery from the unexpected loss of reward relative to the Fischer rats. Pretreatment with the anxiolytic agent, chlordiazepoxide (CDP), effectively eliminated contrast in the Fischer rats, but served to prolong recovery from contrast in the Lewis rats. Finally, the results of Experiment 2 demonstrated that Fischer rats, but not Lewis rats, increase consumption of a 0.1 M sucrose solution following pretreatment with CDP. Together, the results show that, while both Lewis and Fischer rats demonstrate SNC, the effect is more sustained in the Lewis rats and these rats are insensitive to both the anxiolytic and the appetite-stimulating effects of CDP.
Keywords: Contrast effect, Hyperphagia, Reward, Stress
1. Introduction
When a rat is shifted from a strong to a weak reward, consumption of the weaker reward is significantly less than that of a rat that has only been exposed to the weaker reward. This phenomenon, known as successive negative contrast (SNC), was reported in rats in the early 1940s (Crespi, 1944, 1942) and has since received much attention (Flaherty, 1996, 1999; Flaherty et al., 1996; Flaherty and Rowan, 1986; Genn et al., 2004). In the standard paradigm, an unshifted group is given 5 min daily access to a 0.1 M (34.2 g/l) sucrose solution and a shifted group is given 5 min daily access to a preferred 1.0 M (342.3 g/l) sucrose solution. After 10 days, all rats receive 5-min daily access to the weaker sucrose solution across a 4-day postshift period. A SNC effect occurs when rats shifted from the high to the low reward consume significantly less of the low reward than the unshifted controls on the first postshift day (i.e., day 11). Recovery from contrast occurs as intake returns to the level of the unshifted controls over the 4-day postshift period.
A multistage model of negative contrast has been proposed (Flaherty, 1999) which incorporates exploratory and emotional interpretations of the phenomenon. This model states that a rat transitions through several processes in response to the loss of reward in the SNC paradigm. These stages can be divided into cognitive and emotional components. The cognitive component consists of the development of a preshift concentration representation; detection of the postshift concentration; evaluation of the lesser concentration compared to the memory of the previously received tastant; a search for the missing reward; and, once having determined that the lesser postshift solution is the only solution available, acceptance of the lesser reward. In support, Flaherty (1999) provided evidence that rats detect the lesser reward within the first bout of licks, after which intake precipitously declines. Microstructural analysis shows that shifted rats initiate more drinking bursts, but fewer licks per burst, than unshifted rats (Grigson et al., 1993), indicating that a shifted rat repeatedly initiates intake of the less preferred postshift solution, but stops intake soon after initiation. Flaherty (1999) also showed that, when downshifted in a radial arm maze, rats literally ‘search’ for the missing reward.
In addition to the cognitive component, evidence indicates that SNC also involves an emotional process akin to anxiety. That is, conflict is thought to ensue as the hungry rat comes to accept a lesser reward than that which was expected (Becker et al., 1984; Flaherty and Rowan, 1989, 1986). In accordance, SNC involves an increase in corticosterone levels on the second postshift day (Flaherty et al., 1985; Mitchell and Flaherty, 1998) and recovery from the loss of reward is facilitated by pretreatment with the anxiolytic agent, chlordiazepoxide (CDP), in a dose and time dependent manner (Flaherty, 1990; Flaherty et al., 1990, 1980; Rosen and Tessel, 1970). Specifically, benzodiazepine pretreatment reduces contrast when administered on the 2nd, but not on the 1st postshift day (Flaherty et al., 1990, 1980). Together these data led Flaherty (1999) to hypothesize that the loss of reward elicits an emotional component which is associated with an increase in analgesic/anxiolytic steroids that activate an, as of yet, uncharacterized GABAergic system. Activation of this GABAergic system is thought to facilitate recovery from the loss of reward and to allow for the effectiveness of anxiolytic agents on the 2nd postshift day. Recovery generally occurs by the 3rd postshift day.
Lewis rats have been labeled reward-preferring compared with the relatively reward-insensitive Fischer rats. As evidence, it has been demonstrated that Lewis rats show greater place preference for a location paired with a drug of abuse (Guitart et al., 1992; Kosten et al., 1994), they more readily self-administer drugs of abuse compared with Fischer rats (Ambrosio et al., 1995; Kosten et al., 1997), and exhibit higher break point for access to morphine when tested on a progressive ratio schedule of reinforcement (Martin et al., 2003, 1999). In addition, Lewis rats also have been shown to be more sensitive to methamphetamine-induced and cocaine-induced reinstatement compared with Fischer rats (Kruzich and Xi, 2006a,b). Recent evidence, however, has demonstrated that Lewis and Fischer rats do not differ in progressive ratio responding (i.e., in break points) for a natural reward such as food (Martin et al., 1999).
If Lewis rats are truly reward-preferring, we would predict that SNC effects may be greater in Lewis rats than in Fischer rats. However, regardless of their reward-preferring status, larger SNC effects also can be predicted for the Lewis rats on the basis of their performance in a related paradigm, anticipatory contrast. Anticipatory contrast, like SNC, involves the comparison of two rewards and the effect that this comparison has on subsequent intake. In the standard anticipatory contrast paradigm, rats reduce intake of a lesser valued saccharin cue as it comes to predict, via once daily pairings, subsequent availability to a highly preferred 1.0 M sucrose solution (for a review, see Flaherty, 1999). Evidence suggests that these phenomena depend upon similar neural substrates, as both SNC and anticipatory contrasts depend upon an intact gustatory thalamus (Reilly et al., 2004; Reilly and Trifunovic, 2003; Schroy et al., 2005). Anticipatory contrast effects have been found to be larger in Lewis than in Fischer rats (Grigson and Freet, 2000). Thus, we hypothesize that Lewis rats also will demonstrate larger SNC as well. Finally, it also is possible that Fischer rats may recover faster from the unexpected loss of reward due to larger corticosterone responses observed in this strain (Dhabhar et al., 1993; Glowa et al., 1992; Stohr et al., 2000). This larger corticosterone response may activate the GABAergic system to a greater degree, which would, according to Flaherty (1999), result in faster recovery from the loss of reward in Fischer 344 rats compared to Lewis rats. The following experiments were conducted to explore the validity of these predictions.
2. Experiment 1
As previously stated, Experiment 1 was designed to test whether Lewis rats would exhibit greater SNC effects following the loss of reward relative to the less reward-sensitive Fischer rat. Lewis and Fischer rats were compared across four variables: the presence of successive negative contrast, the magnitude of the negative contrast effect, the rate of recovery from the loss of reward, and the effectiveness of the anxiolytic agent, CDP, on recovery.
2.1. Methods
2.1.1. Subjects
The study was run in two separate replications, an initial pilot study and a larger replication. With the data collapsed, the subjects were 34 male Fischer 344 rats (Harlan Laboratories) and 36 male Lewis rats (Harlan Laboratories), with the Fischer rats weighing between 189 and 238 g and the Lewis rats weighing between 214 and 281 g at the beginning of the experiment. They were housed individually in stainless steel hanging cages in a temperature-controlled (21 °C) animal care facility with a 12:12 h light:dark cycle (lights on at 7:00 am). All experimental manipulations were conducted 3 h into the light phase of the cycle. The rats were maintained with free access to dry Harlan Teklad rodent diet and water except where otherwise noted. An Institutional Review Committee for the use of Animal Subjects approved the experimental protocol and the procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985).
2.1.2. Apparatus
The rats were trained in one of the six identical modular operant chambers (MED Associates, Inc., St. Albans, VT) measuring 30.5×24.0×29.0 cm (length×width×height). All chambers had clear Plexiglas top, front, and back walls, while the sidewalls were made of aluminum. The grid floors consisted of nineteen 4.8 mm stainless steel rods spaced 1.6 cm apart (center to center). Each chamber was equipped with a retractable sipper tube that could enter the chamber through a 1.3 cm diameter hole. In the extended position, the tip of the sipper tube was aligned in the center of the hole, flush with the right end wall. A lickometer circuit was used to monitor licking. A shaded bulb, which reflected light off the ceiling, was located on the right of the cage and the white noise speaker which provided a background noise level of 75 dB(A) was located on the left-end wall, opposite to the sipper tubes. Each chamber was housed in a light and sound attenuated cubicle that was fitted with a ventilation fan. Control of events in the chamber and collection of the data were carried out on-line with a 90-MHz computer. Programs were written in the Medstate notation language (MED Associates, Inc., St. Albans, VT 05478).
2.1.3. Procedure
Prior to the experiment, rats were food deprived to 82% of their free-feeding body weight, maintained by a once per day feeding. The Lewis and Fischer rats were divided into two groups (unshifted and shifted). The unshifted group (n =18 Lewis, n =17 Fischer) was given 5 min access to 0.1 M sucrose daily for 14 days. The shifted group (n =18 Lewis, n =17 Fischer) was given 5 min access to 1.0 M sucrose daily for 10 days and then was shifted to 0.1 M sucrose on days 11–14. On postshift day 2 (or day 12), half of the shifted and unshifted rats (shifted: n =9 Lewis, n =8 Fischer; unshifted: n =9 Lewis, n =8 Fischer) were injected intraperitoneally (ip) with 0.9% saline 30–35 min prior to being placed into the test chamber. The other half of the rats (shifted: n =9 Lewis, n =9 Fischer; unshifted: n =9 Lewis, n =9 Fischer) were injected ip with a 10 mg/kg dose of CDP. The 10 mg/kg dose of CDP was selected because it has been shown to be highly effective in reducing SNC effects when administered on the 2nd postshift day in Sprague–Dawley rats (Flaherty et al., 1990).
Sucrose (saccharose), obtained from Fisher Chemical, Pittsburgh, Pennsylvania, was prepared 24 h in advance and presented at room temperature. Chlordiazepoxide was obtained from the Sigma Chemical Company, St. Louis, Missouri and was mixed in 0.9% saline 1–2 h before administration.
2.2. Results and discussion
To examine the preference for sucrose within and between the strains, the intake data for the first 10 trials were analyzed using a 2×2×10 mixed factorial ANOVA varying strain, sucrose concentration, and trials. Post hoc Newman–Keuls tests on the significant 3-way interaction, F (9486)=1.95, p =.04, revealed that Lewis rats made more licks for 1.0 M sucrose on all preshift days (trials 1–10) than did Fischer rats, ps<0.001; Lewis rats also drank more 0.1 M sucrose than Fischer rats, ps>0.03. Prior to the shift, Lewis rats drank more 1.0 M than 0.1 M sucrose on all trials, ps<.004. Fischer rats also made more licks for 1.0 M than 0.1 M sucrose on all trials (p <.005) except trial 9 (p =.06) and trial 10 (p =.07) (Fig. 1).
Fig. 1.
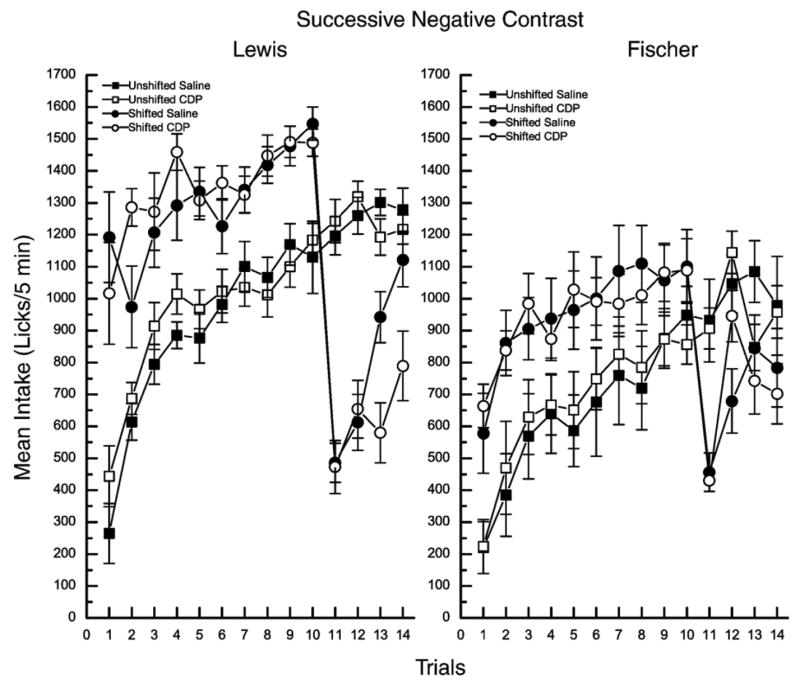
Mean intake for the preshift days (trials 1–10) and postshift days (trials 11–14) for Lewis (left panel) and Fischer (right panel) rats. In both strains, half of the rats were given 5 min access to 0.1 M sucrose throughout the study (unshifted, squares). The other half of the rats were given 5 min access to 1.0 M sucrose for 10 trials and were then downshifted to 0.1 M sucrose across trials 11–14 (shifted, circles). On trial 12, half of the rats from each shift condition were pretreated with saline (closed symbols) and the other half with chlordiazepoxide (CDP, open symbols).
To evaluate SNC, the intake data were analyzed using a 2×2×2×2×14 mixed factorial ANOVA varying replication, strain, shift, drug, and trials. As the 5-way interaction just missed significance, F (13,702)=1.71, p>.05, the replication factor was omitted and the data were reanalyzed using a 2×2×2×14 mixed factorial ANOVA. The results showed that the 4-way strain×shift×drug×trials interaction was significant, F (13,702)=1.79, p<.04. On the day of the shift (trial 11), both strains demonstrated successive negative contrast as defined by a significant decrease in intake from trial 10 to trial 11 (shift magnitude, ps<.0001) and a significant decrease in intake relative to their respective unshifted controls (contrast magnitude, ps<.0002) (Fig. 1). In addition, a shift ratio was calculated for the shifted group in each strain (trial 11/trial 10). The data were collapsed across drug treatment for each strain, as the drug was not presented until trial 12. The shift ratios were then compared using independent t-tests. The results of this analysis showed that the Lewis rats exhibited a smaller shift ratio (i.e., a larger shift) than the Fischer rats (Lewis=0.32 (±.03); Fischer=0.42 (±.03) and this difference was statistically significant, (t (33)=2.42, p<.01). Taken together, these data show that the unexpected loss of reward led to contrast in both strains of rats and the magnitude of this effect tended to be larger in the reward-sensitive Lewis rats.
As indicated by examination of the data for the vehicle-treated controls, recovery from the loss of reward also was slower in the Lewis, relative to the Fischer, rats. Shifted Fischer rats recovered from contrast by the 3rd postshift day (i.e., intake by the shifted Fischer rats did not differ from that of their unshifted controls by trial 13, p=.73). Shifted Lewis rats, on the other hand, continued to consume less of the 0.1 M sucrose solution than their unshifted controls on trial 13, p<.05, and did not recover from reward downshift until trial 14, p =.90. In the Fischer rats, the administration of CDP on trial 12 led to a complete recovery of contrast relative to unshifted CDP-treated controls, p=.93. A single exposure to the same dose of the drug, however, exerted no contrast-reducing effect in the shifted Lewis rats. Intake of the 0.1 M sucrose solution by the CDP-treated shifted Lewis rats remained below that of their unshifted CDP, p<.0001, or saline, p<.0001, treated controls and statistically similar to the shifted saline group, p=.99. Finally, for Lewis rats, the administration of CDP served to prevent recovery from the unexpected loss of reward over the 4-day postshift period. Thus, the shifted CDP-treated Lewis rats made fewer licks for the lesser postshift solution than the unshifted controls on trial 13 and on trial 14 as well, ps<.0001.
In addition to anxiolytic effects, CDP, and benzodiazepines in general, also induce the known appetite-stimulating effects (Berridge and Treit, 1986; Flaherty, 1999). Despite these rather potent effects, CDP supported only a small trend toward an appetite-stimulating effect in the unshifted Fischer rats and no appetite-stimulating effect at all in the unshifted Lewis rats. These data may indicate that both strains of rats are insensitive to the appetite-stimulating effects of CDP. Alternatively, Fischer rats may be more sensitive than Lewis rats, but the magnitude of the effect may have been occluded in the Fischer rats by a ceiling effect. Thus, Fischer rats may have been making their maximum number of licks in the 5-min test session, and licking could not be further augmented by the administration of CDP. Experiment 2 will eliminate this potential confound to better evaluate the appetite-stimulating effect of CDP in both strains of rat.
3. Experiment 2
The results of Experiment 1 showed that both Lewis and Fischer rats demonstrate successive negative contrast effects. The magnitude of this effect tended to be larger in Lewis rats and Lewis rats also were slower to recover from the loss of expected reward. In addition, while the administration of CDP facilitated the rate of recovery in Fischer rats on postshift day 2, it failed to do so in Lewis rats. In fact, the rate of recovery in Lewis rats was delayed following the administration of the drug. In addition to these findings, the results of Experiment 1 also showed that CDP-induced a small, non-significant appetite-stimulating effect in the Fischer rats and no appetite-stimulating effect what-so-ever in the Lewis rats. Experiment 2 revisited this issue in naïve Lewis and Fischer rats using the procedures described by Twining et al. (2005). Specifically, the rats were tested in a non-deprived state so that ceiling effects could not impede the data for either the Lewis or the Fischer rats. The 10 mg/kg dose of CDP was used, as this dose has been found to support robust appetite-stimulating effects in Sprague–Dawley rats using identical procedures (Twining et al., 2005).
3.1. Methods
3.1.1. Subjects
The subjects were 12 naïve male Fischer rats (Harlan Laboratories) and 12 naïve male Lewis rats (Harlan Laboratories) with the Fischer rats weighing between 207 and 230 g and the Lewis rats weighing between 216 and 244 g at the beginning of the experiment. All rats were housed individually in stainless steel hanging cages in a temperature-controlled (21 °C) animal care facility with a 12:12 h light–dark cycle (lights on at 7:00 am). All experimental manipulations were begun 5.5 to 6.5 h into the light phase of the cycle (12:30 pm to 1:30 pm). The rats were maintained with free access to dry Harlan Teklad rodent diet and water, except where otherwise noted. An Institutional Review Committee for the use of Animal Subjects approved the experimental protocol and the procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985).
3.1.2. Apparatus
The apparatus was the same as that described in Experiment 1.
3.1.3. Procedure
Across trials 1–12, each subject was taken from his home cage, weighed, and placed in the module. Immediately after initiating the trial, the house light came on and a bottle containing a 0.1 M sucrose solution was advanced. Each subject was given 5 min access to this bottle at which time the bottle was retracted, the house light shut off, and the subject removed from the box and placed back in the home cage. On trials 6, 7, 9, 10, and 12, each subject received an ip injection of 0.9% saline 30–35 min prior to run time. On trials 8 and 11, each subject received an ip injection of the 10 mg/kg dose of CDP 30–35 min prior to the start of the experimental session.
Sucrose and CDP were obtained and prepared as described in Experiment 1.
3.2. Results and discussion
The benzodiazepine, CDP, induced significant appetite-stimulating effects in Fischer, but not in Lewis, rats (see Fig. 2). This observation was confirmed by a 2×12 mixed factorial ANOVA which demonstrated a significant strain×trials interaction, F (11,242) = 4.03, p <.0001, as well as significant main effects of strain, F (122)=41.98, p<.0001, and trials, F (11,242)=16.88, p<.0001. Newman–Keuls post hoc comparisons of the significant strain×trials interaction demonstrated that, in Lewis rats, there was no significant difference between intake on trial 8 (CDP) and trial 7 (saline; p=.54) or trial 9 (saline; p=.48) or between trial 11 (CDP) and trial 10 (saline; p=.57) or trial 12 (saline; p=.68). Intake by the Fischer rats, however, was significantly different across these comparisons. The number of licks on trial 8 (CDP) was greater than that generated on trial 7 (saline; p=.02) and trial 9 (saline; p=.01) and the number of licks emitted on trial 11 (CDP) was greater than the number emitted on trial 10 (saline; p=.0001) and trial 12 (saline; p=.001). These data confirm that CDP induces potent appetite-stimulating effects in the Fischer rats, but is fully ineffective in the Lewis rats.
Fig. 2.
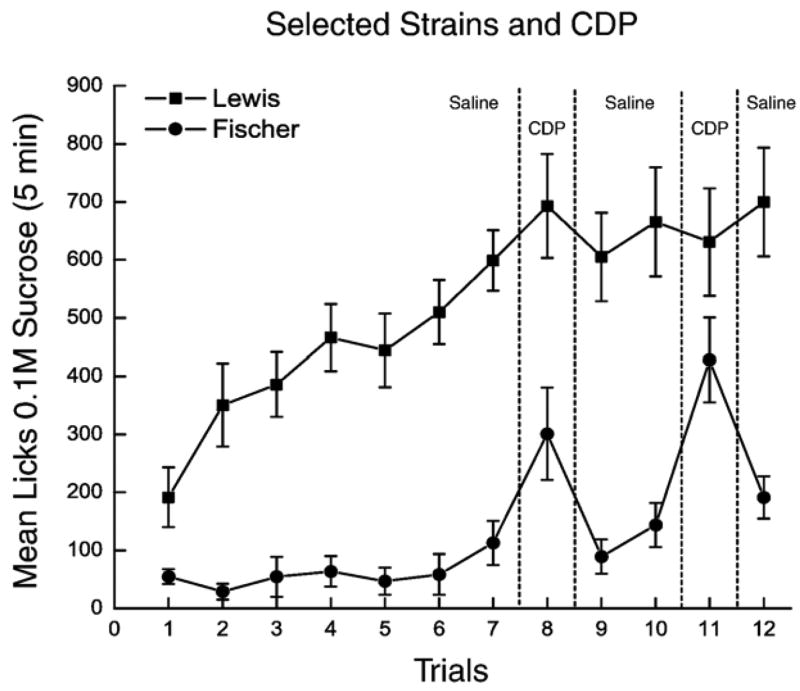
Mean intake for Lewis (closed square) and Fischer (open circle) rats is presented for trials 1–12. All rats received an ip injection of saline on trials 7, 9, 10, and 12 and 10 mg/kg CDP on trials 8 and 11.
4. General discussion
The data from Experiments 1 and 2 demonstrate that Lewis and Fischer rats both show successive negative contrast effects, yet they respond differently on two other major indices: rate of recovery and in response to CDP (i.e., to both the anxiolytic and appetite-stimulating effects of the drug). The data from Experiment 1 clearly show that both the Lewis and the Fischer rats exhibit robust SNC effects relative to their own unshifted controls. The magnitude of the drop in intake is larger in the Lewis rats (as indicated by the shift ratio), but the interpretation of this finding is not straightforward. That is, while the Fischer rats displayed a smaller reduction in intake when shifted to the lesser reward, it is obvious that these rats also made fewer licks for the 1.0 M preshift solution than did the Lewis rats. Ceiling effects, then, may have contributed to the smaller contrast effect (i.e., to the higher shift ratio) in the Fischer rats. Even so, it is also clear that the shifted Fischer rats could have made fewer licks than 400 during the first 5-min postshift period, but they did not. Thus, when taken together, the data demonstrate that, relative to Fischer rats, SNC effects tend to be larger in Lewis rats following the unexpected loss of reward. Future studies may revisit this issue using longer access to the preshift solution. Acquisition in various paradigms has been shown to be slower in Fischer than Lewis rats (Kosten and Ambrosio, 2002; Kosten et al., 1997). Manipulations known to support smaller contrast effects also may be implemented because strain differences in the anticipatory contrast paradigm were revealed between the Lewis and Fischer rats only when using procedures that supported small anticipatory contrast effects (Grigson and Freet, 2000).
The other major finding was the differential effects of the common anxiolytic agent CDP. In Experiment 1, CDP failed to attenuate contrast in the Lewis rats. This dose, which facilitated recovery in the Fischer rats, is also known to facilitate recovery in outbred rats in a dose and time dependent manner when administered on the second postshift day (Flaherty, 1990; Flaherty et al., 1990, 1980; Rosen and Tessel, 1970). In general, this finding is consistent with the literature. Lewis rats are reportedly less sensitive than Fischer rats to benzodiazepines in several other paradigms including the development of physical dependence to diazepam, elevated plus-maze performance, and the anxiolytic-like effect of the NK-1 receptor antagonist NKP608 (Suzuki et al., 1992; Takahashi et al., 2001; Vendruscolo et al., 2003). Pre-treatment with CDP, however, not only failed to facilitate recovery, but actually retarded recovery from the loss of reward. This is of interest as it suggests that CDP may activate a mechanism that directly opposes recovery from the loss of reward.
In Experiment 2, differences in CDP-induced hyperphagia were also observed between Lewis and Fischer rats. Again, a review of the literature suggests that failure to demonstrate CDP-induced appetite-stimulating effects by the Lewis rats is not due to a general failure to exhibit drug-induced appetite-stimulating effects, per se, but to a more specific failure to respond to the benzodiazepine. Thus, Lewis rats have been shown to exhibit larger, rather than smaller, appetite-stimulating effects following the administration of morphine (Gosnell and Krahn, 1993) and they exhibit clear appetite-stimulating effects following the administration of Delta(9)-THC (Koch, 2001). Lewis rats, then, are less sensitive than Fischer rats to a range of effects associated with the administration of benzodiazepine.
In sum, it has been demonstrated previously that Lewis and Fischer rats behave differently in a reward comparison paradigm, namely anticipatory contrast (Grigson and Freet, 2000). Here we have extended this finding to include another reward comparison paradigm, successive negative contrast. While it currently is uncertain whether Lewis rats differ from Fischer rats in the magnitude of the shift, marked strain differences were found in the rate of recovery as well as in the anxiolytic and appetite-stimulating effects of CDP. Relative to saline-treated Fischer rats, saline-treated Lewis rats showed a slower rate of recovery from contrast. As occurs with Sprague–Dawley rats (Flaherty, 1999), pretreatment with CDP served to eliminate contrast when administered on the 2nd postshift day in Fischer 344 rats. Pretreatment with CDP in Lewis rats, on the other hand, failed to attenuate contrast and ultimately served to delay recovery. Lewis rats also were found to be fully insensitive to the appetite-stimulating effects of the benzodiazepine, while non-deprived Fischer rats demonstrated a profound hyperphagic response to CDP. Published data show that this effect relates to a specific failure to appropriately respond to the benzodiazepine, rather than to a general failure to exhibit appetite-stimulating effects, per se. Additional studies are required to determine whether these strain differences in behavior are due to known strain differences in the responsiveness of the HPA axis and/or in the GABA/benzodiazepine chloride ionophore.
Acknowledgments
This work was supported by NIH grants DA09815 and DA12473.
References
- Ambrosio E, Goldberg SR, Elmer GI. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol. 1995;6:229–37. [PubMed] [Google Scholar]
- Becker HC, Jarvis MF, Wagner GC, Flaherty CF. Medial and lateral amygdalectomy differentially influences consummatory negative contrast. Physiol Behav. 1984;33:707–12. doi: 10.1016/0031-9384(84)90035-0. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Treit D. Chlordiazepoxide directly enhances positive ingestive reactions in rats. Pharmacol Biochem Behav. 1986;24:217–21. doi: 10.1016/0091-3057(86)90341-2. [DOI] [PubMed] [Google Scholar]
- Crespi LP. Quantitative variation in incentive and performance in the white rat. Am J Psychol. 1942;55:467–517. [Google Scholar]
- Crespi LP. Amount of reinforcement and level of performance. Psychol Rev. 1944;51:341–57. [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels— a comparison between Sprague–Dawley, Fischer 344 and Lewis rats. Brain Res. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Effect of anxiolytics and antidepressants on extinction and negative contrast. Pharmacol Ther. 1990;46:309–20. doi: 10.1016/0163-7258(90)90097-l. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Incentive Relativity. New York: Cambridge University Press; 1996. [Google Scholar]
- Flaherty CF. Incentive Relativity. New York: Cambridge University Press; 1999. [Google Scholar]
- Flaherty CF, Rowan GA. Successive, simultaneous, and anticipatory contrast in the consumption of saccharin solutions. J Exp Psychol Anim Behav Processes. 1986;12:381–93. [PubMed] [Google Scholar]
- Flaherty CF, Rowan GA. Rats (Rattus norvegicus) selectively bred to differ in avoidance behavior also differ in response to novelty stress, in glycemic conditioning, and in reward contrast. Behav Neural Biol. 1989;51:145–64. doi: 10.1016/s0163-1047(89)90782-6. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Lombardi BR, Wrightson J, Deptula D. Conditions under which chlordiazepoxide influences gustatory contrast. Psychopharmacology (Berl) 1980;67:269–77. doi: 10.1007/BF00431269. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Becker HC, Pohorecky L. Correlation of corticosterone elevation and negative contrast varies as a function of postshift day. Anim Learn Behav. 1985;13:309–14. [Google Scholar]
- Flaherty CF, Grigson PS, Lind S. Chlordiazepoxide and the moderation of the initial response to reward reduction. Q J Exp Psychol B. 1990;42:87–105. [PubMed] [Google Scholar]
- Flaherty CF, Coppotelli C, Potaki J. Effect of chlordiazepoxide on the response to repeated reductions in sucrose concentration in free-fed rats. Physiol Behav. 1996;60:1291–8. doi: 10.1016/s0031-9384(96)00257-0. [DOI] [PubMed] [Google Scholar]
- Genn RF, Ahn S, Phillips AG. Attenuated dopamine efflux in the rat nucleus accumbens during successive negative contrast. Behav Neurosci. 2004;118:869–73. doi: 10.1037/0735-7044.118.4.869. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Geyer MA, Gold PW, Sternberg EM. Differential startle amplitude and corticosterone response in rats. Neuroendocrinology. 1992;56:719–23. doi: 10.1159/000126298. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Krahn DD. Morphine-induced feeding: a comparison of the Lewis and Fischer 344 inbred rat strains. Pharmacol Biochem Behav. 1993;44:919–24. doi: 10.1016/0091-3057(93)90025-o. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Freet CS. The suppressive effects of sucrose and cocaine, but not lithium chloride, are greater in Lewis than in Fischer rats: evidence for the reward comparison hypothesis. Behav Neurosci. 2000;114:353–63. doi: 10.1037//0735-7044.114.2.353. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Spector AC, Norgren R. Microstructural analysis of successive negative contrast in free-feeding and deprived rats. Physiol Behav. 1993;54:909–16. doi: 10.1016/0031-9384(93)90301-u. [DOI] [PubMed] [Google Scholar]
- Guitart X, Beitner-Johnson D, Marby DW, Kosten TA, Nestler EJ. Fischer and Lewis rat strains differ in basal levels of neurofilament proteins and their regulation by chronic morphine in the mesolimbic dopamine system. Synapse. 1992;12:242–53. doi: 10.1002/syn.890120310. [DOI] [PubMed] [Google Scholar]
- Koch JE. Delta(9)-THC stimulates food intake in Lewis rats: effects on chow, high-fat and sweet high-fat diets. Pharmacol Biochem Behav. 2001;68:539–43. doi: 10.1016/s0091-3057(01)00467-1. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneur-oendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–44. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–29. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Xi J. Differences in extinction responding and reinstatement of methamphetamine-seeking behavior between Fischer 344 and Lewis rats. Pharmacol Biochem Behav. 2006a;83:391–5. doi: 10.1016/j.pbb.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Xi J. Different patterns of pharmacological reinstatement of cocaine-seeking behavior between Fischer 344 and Lewis rats. Psychopharmacology (Berl) 2006b;187:22–9. doi: 10.1007/s00213-005-0264-4. [DOI] [PubMed] [Google Scholar]
- Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–5. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Martin S, Lyupina Y, Crespo JA, Gonzalez B, Garcia-Lecumberri C, Ambrosio E. Genetic differences in NMDA and D1 receptor levels, and operant responding for food and morphine in Lewis and Fischer 344 rats. Brain Res. 2003;973:205–13. doi: 10.1016/s0006-8993(03)02482-x. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Flaherty C. Temporal dynamics of corticosterone elevation in successive negative contrast. Physiol Behav. 1998;64:287–92. doi: 10.1016/s0031-9384(98)00072-9. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Gustatory thalamus lesions eliminate successive negative contrast in rats: evidence against a memory deficit. Behav Neurosci. 2003;117:606–15. doi: 10.1037/0735-7044.117.3.606. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova M, Trifunovic R. Excitotoxic lesions of the gustatory thalamus spare simultaneous contrast effects but eliminate anticipatory negative contrast: evidence against a memory deficit. Behav Neurosci. 2004;118:365–76. doi: 10.1037/0735-7044.118.2.365. [DOI] [PubMed] [Google Scholar]
- Rosen AJ, Tessel RE. Chlorpromazine, chlordiazepoxide, and incentive-shift performance in the rat. J Comp Physiol Psychol. 1970;72:257–62. doi: 10.1037/h0029467. [DOI] [PubMed] [Google Scholar]
- Schroy PL, Wheeler RA, Davidson C, Scalera G, Twining RC, Grigson PS. Role of gustatory thalamus in anticipation and comparison of rewards over time in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R966–80. doi: 10.1152/ajpregu.00292.2004. [DOI] [PubMed] [Google Scholar]
- Stohr T, Szuran T, Welzl H, Pliska V, Feldon J, Pryce CR. Lewis/Fischer rat strain differences in endocrine and behavioural responses to environmental challenge. Pharmacol Biochem Behav. 2000;67:809–19. doi: 10.1016/s0091-3057(00)00426-3. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Lu MS, Motegi H, Yoshii T, Misawa M. Genetic differences in the development of physical dependence upon diazepam in Lewis and Fischer 344 inbred rat strains. Pharmacol Biochem Behav. 1992;43:387–93. doi: 10.1016/0091-3057(92)90167-e. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Berton O, Mormede P, Chaouloff F. Strain-dependent effects of diazepam and the 5-HT2B/2C receptor antagonist SB 206553 in spontaneously hypertensive and Lewis rats tested in the elevated plus-maze. Braz J Med Biol Res. 2001;34:675–82. doi: 10.1590/s0100-879x2001000500017. [DOI] [PubMed] [Google Scholar]
- Twining RC, Hajnal A, Han L, Bruno K, Hess EJ, Grigson PS. Lesions of the ventral tegmental area disrupt drug-induced appetite stimulating effects but spare reward comparison. Int J Comp Psychol. 2005;18:372–96. [Google Scholar]
- Vendruscolo LF, Takahashi RN, Bruske GR, Ramos A. Evaluation of the anxiolytic-like effect of NKP608, a NK1-receptor antagonist, in two rat strains that differ in anxiety-related behaviors. Psychopharmacology (Berl) 2003;170:287–93. doi: 10.1007/s00213-003-1545-4. [DOI] [PubMed] [Google Scholar]


