Abstract
Mice pretreated with cyclophosphamide were reconstituted with syngeneic cells. Their response to pneumococcal polysaccharide type III (S III) was considered to measure the activity of transferred B cells and the response to sheep or horse erythrocytes (SRBC and HRBC) that of B and T cells.
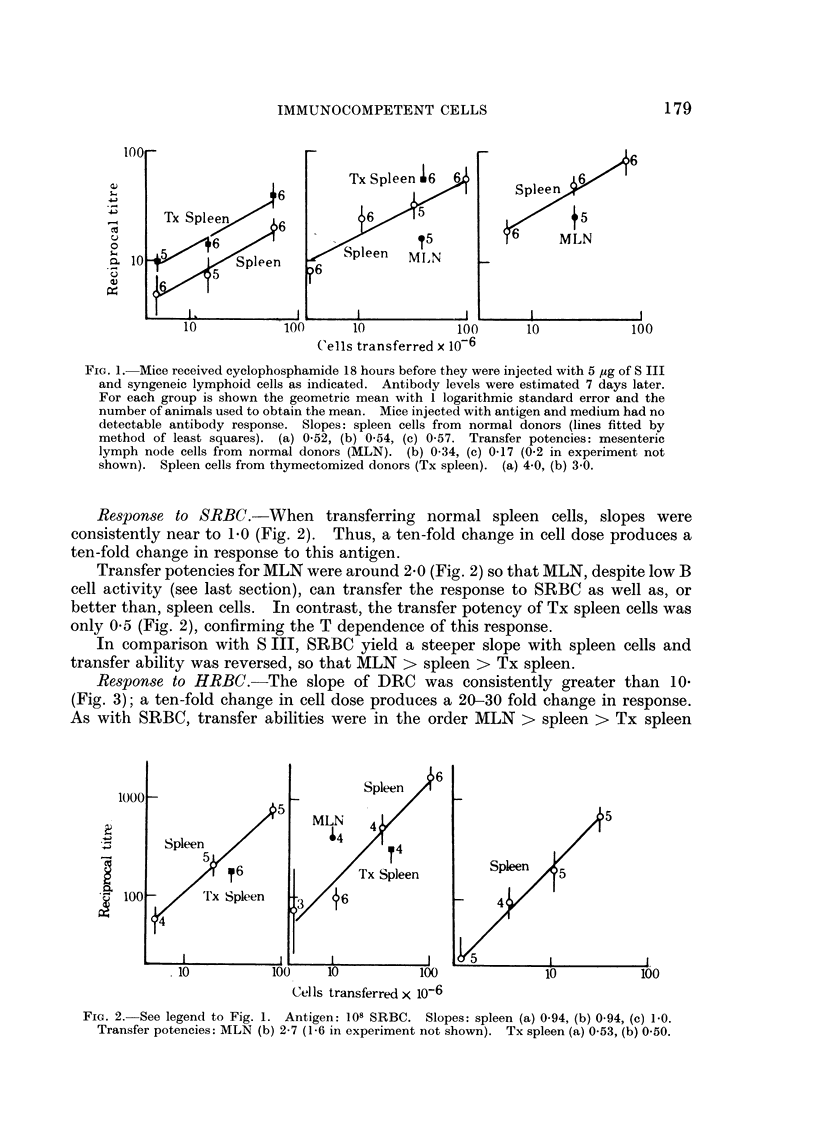
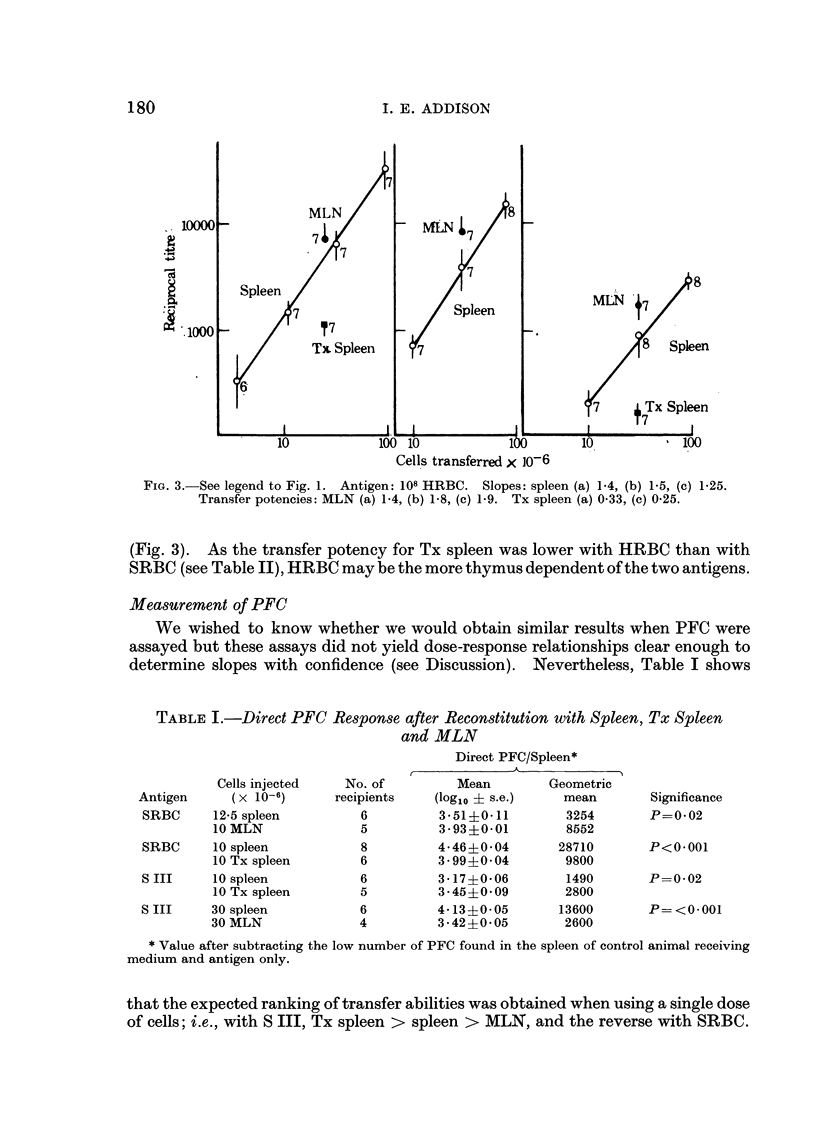
The populations of donor cells used contained different proportions of T and B cells; their response to S III ranked in the order, spleen from thymectomized donors > normal spleen > lymph node; the reverse obtained in the responses to erythrocytes. Thus, lymph node suspensions made the most antibody to thymus dependent antigens though they contained the least B cell activity. It is concluded that T cells, rather than initiating, amplify the response of B cells to these antigens.
Spleen cells were used to obtain dose response curves for each antigen. A ten-fold increase in cell dose increased the response to S III three-fold, to SRBC ten-fold, and to HRBC twenty-fold or more. These differences follow if amplification of the B cell response increases with T cell dose, and is greater for HRBC than SRBC.
It is argued that agents which alter the proportions of B and T cells equally, may not affect thymus dependent and independent responses equally.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison I. E. Immunosuppression with busulphan: the effect on spleen, marrow and thymus cells of mice. Eur J Immunol. 1973 Jul;3(7):419–424. doi: 10.1002/eji.1830030709. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Davies A. J. Early affect of adult thymectomy. Nat New Biol. 1971 May 26;231(21):110–111. doi: 10.1038/newbio231110a0. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Muller J. Y., Dardenne M. In vivo specific antigen recognition by rosette forming cells. Nature. 1970 Sep 19;227(5264):1251–1252. doi: 10.1038/2271251a0. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Butterworth A. E. Susceptibility of human and murine lymphocytes and thymocytes to damage by hypotonic shock. Immunology. 1971 Oct;21(4):701–709. [PMC free article] [PubMed] [Google Scholar]
- Cohn M. Immunology: what are the rules of the game? Cell Immunol. 1972 Sep;5(1):1–20. doi: 10.1016/0008-8749(72)90079-2. [DOI] [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V., Dietrich F. M. The morphology of immune reactions in normal, thymectomized and reconstituted mice. 3. Response to bacterial antigens: salmonellar flagellar antigen and pneumococcal plysaccharide. Immunology. 1970 Dec;19(6):945–957. [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W. The role of T cells and adjuvant in the immune response of mice to foreign erythrocytes. Eur J Immunol. 1972 Feb;2(1):50–57. doi: 10.1002/eji.1830020111. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Nossal G. J. Tolerance, enhancement and the regulation of interactions between T cells, B cells and macrophages. Transplant Rev. 1972;13:3–34. doi: 10.1111/j.1600-065x.1972.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Gregory C. J., Lajtha L. G. Kinetic study of the production of antibody-forming cells from their precursors. Nature. 1968 Jun 15;218(5146):1079–1081. doi: 10.1038/2181079a0. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H., PARROTT D. M., EAST J. STUDIES ON GLOBULIN AND ANTIBODY PRODUCTION IN MICE THYMECTOMIZED AT BIRTH. Immunology. 1964 Jul;7:419–439. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M., Leuchars E., Davies A. J. Studies on immunological paralysis. VI. Thymic-independence of tolerance and immunity to type 3 pneumococcal polysaccharide. Cell Immunol. 1971 Dec;2(6):614–626. doi: 10.1016/0008-8749(71)90009-8. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M. Studies on immunological paralysis. IV. The relative contributions of continuous antibody neutralization and central inhibition to paralysis with type 3 pneumococcal polysaccharide. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):417–438. doi: 10.1098/rspb.1971.0073. [DOI] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Variable effects of anti-lymphocyte serum on humoral antibody formation: role of thymus dependency of antigen. J Immunol. 1971 Apr;106(4):917–926. [PubMed] [Google Scholar]
- Krüger J., Gershon R. K. DNA synthetic response of thymocytes to a variety of antigens. J Immunol. 1972 Mar;108(3):581–585. [PubMed] [Google Scholar]
- Marbrook J., Baguley B. C. The recovery of immune responsiveness after treatment with cyclophosphamide. Int Arch Allergy Appl Immunol. 1971;41(6):802–812. doi: 10.1159/000230572. [DOI] [PubMed] [Google Scholar]
- Marbrook J. Primary immune response in cultures of spleen cells. Lancet. 1967 Dec 16;2(7529):1279–1281. doi: 10.1016/s0140-6736(67)90393-5. [DOI] [PubMed] [Google Scholar]
- Miller J. F. Effect of thymectomy in adult mice on immunological responsiveness. Nature. 1965 Dec 25;208(5017):1337–1338. doi: 10.1038/2081337a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Nase S., Mitchison N. A. Mouse specific bone marrow-derived lymphocyte antigen as a marker for thymus-independent lymphocytes. Nature. 1971 Mar 5;230(5288):50–51. doi: 10.1038/230050a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Roelants G. E., Askonas B. A. Carrier specificity and the allogeneic effect in mice. Eur J Immunol. 1972 Dec;2(6):592–598. doi: 10.1002/eji.1830020622. [DOI] [PubMed] [Google Scholar]
- Roitt I. M., Greaves M. F., Torrigiani G., Brostoff J., Playfair J. H. The cellular basis of immunological responses. A synthesis of some current views. Lancet. 1969 Aug 16;2(7616):367–371. doi: 10.1016/s0140-6736(69)92712-3. [DOI] [PubMed] [Google Scholar]
- Roseman J. X-ray resistant cell required for the induction of in vitro antibody formation. Science. 1969 Sep 12;165(3898):1125–1127. doi: 10.1126/science.165.3898.1125. [DOI] [PubMed] [Google Scholar]
- Takasugi M. An improved fluorochromatic cytotoxic test. Transplantation. 1971 Aug;12(2):148–151. doi: 10.1097/00007890-197108000-00010. [DOI] [PubMed] [Google Scholar]


