Abstract
Coxsackie B3 virus injected into mice on the eighth day of pregnancy resulted in foetal wastage and growth retardation. Although in apparent good health, the pregnant animals ate more food than the controls yet failed to increase in body weight as normal. This observation, together with the maternal autopsy findings of pancreatic acinar atrophy and hepatitis, suggests that the animals are subject to a manifestation of dietary deficiency attributable to an inability to break down and digest protein in their diet.
It would seem that whilst the possibility of the virus exerting a direct effect on the foetuses cannot be ignored, the action of the virus in reducing the state of health of the pregnant mother is largely responsible for the foetal effects seen.
Full text
PDF


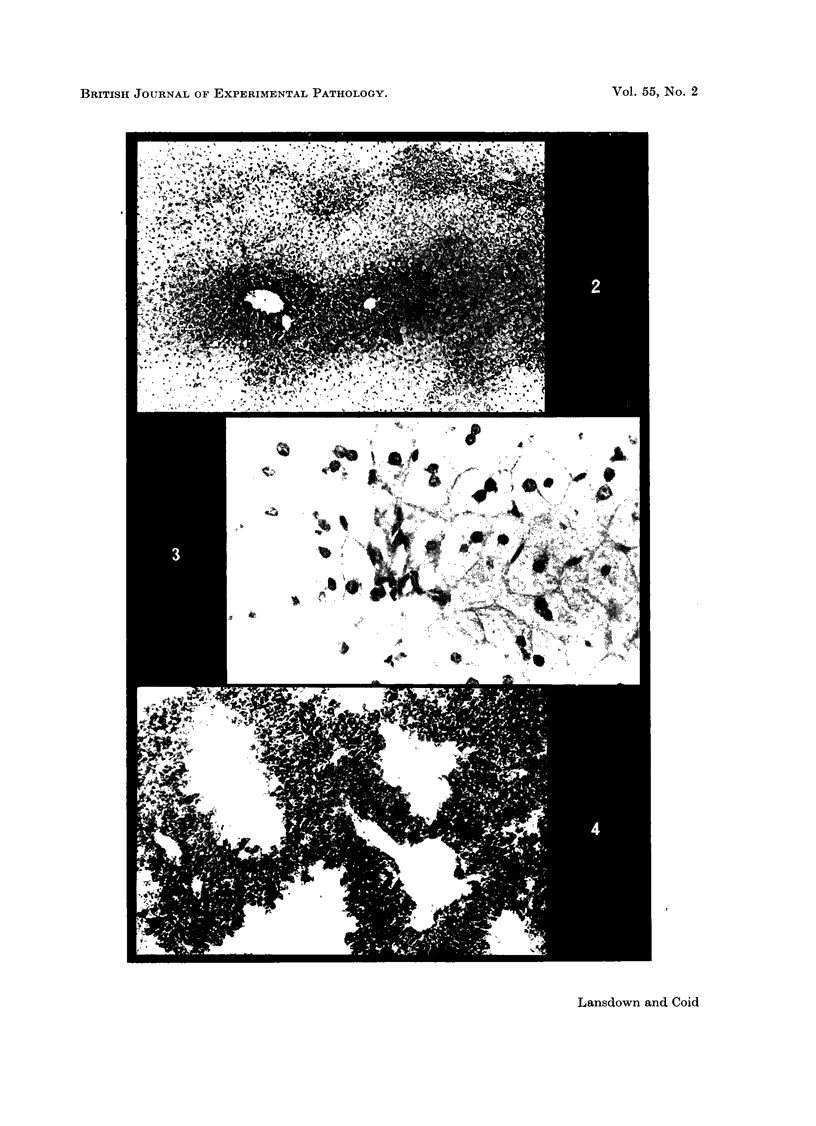
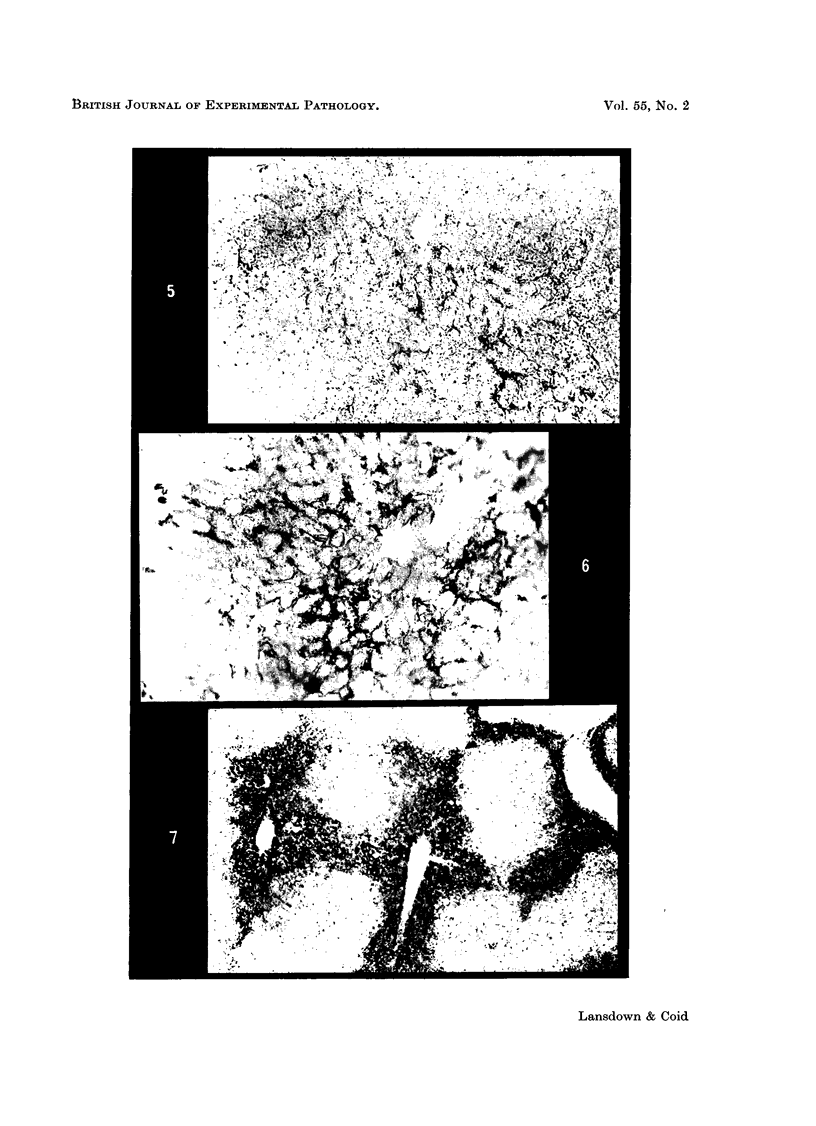
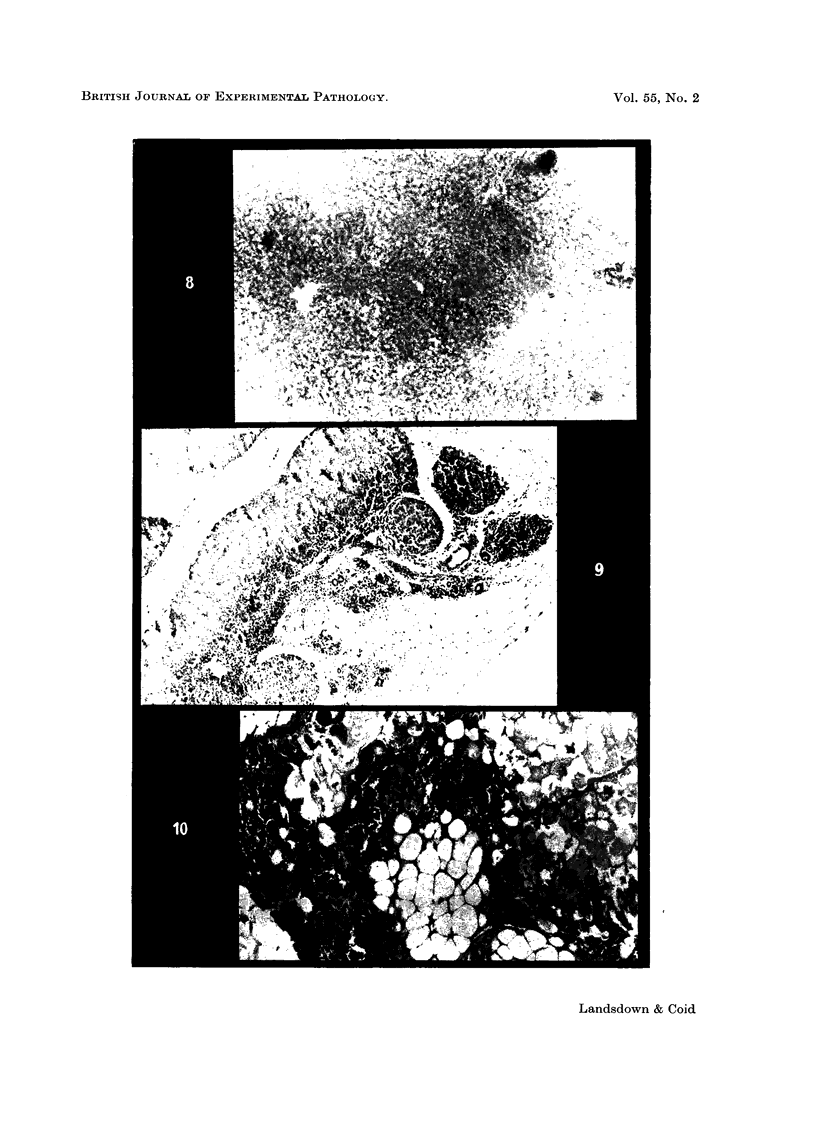





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., BURSTONE M. S. HISTOCHEMICAL DEMONSTRATION OF CHANGES IN LIVER CELL ENZYMES FOLLOWING INFECTION WITH MOUSE HEPATITIS VIRUS. Z Zellforch Microsk Anat Histochem. 1964 Jan 31;48:462–466. doi: 10.1007/BF00736423. [DOI] [PubMed] [Google Scholar]
- Abraham R., Morris M., Hendy R. Lysosomal changes in epithelial cells of the mouse thymus after hydrocortisone treatment. Histochemie. 1969;17(4):295–311. doi: 10.1007/BF00305454. [DOI] [PubMed] [Google Scholar]
- Ballard K. J., Holt S. J. Cytological and cytochemical studies on cell death and digestion in the foetal rat foot: the role of macrophages and hydrolytic enzymes. J Cell Sci. 1968 Jun;3(2):245–262. doi: 10.1242/jcs.3.2.245. [DOI] [PubMed] [Google Scholar]
- Brown G. C., Karunas R. S. Relationship of congenital anomalies and maternal infection with selected enteroviruses. Am J Epidemiol. 1972 Mar;95(3):207–217. doi: 10.1093/oxfordjournals.aje.a121388. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Tsui C. Y., Harb J. M. Hepatitis in mice infected with Coxsackie virus B 1 . Br J Exp Pathol. 1973 Jun;54(3):249–254. [PMC free article] [PubMed] [Google Scholar]
- Burch G. E., Tsui C. Y., Harb J. M. Pancreatic islet cell damage in mice produced by coxsackie B 1 and encephalomyocarditis viruses. Experientia. 1972 Mar 15;28(3):310–311. doi: 10.1007/BF01928709. [DOI] [PubMed] [Google Scholar]
- Catalano L. W., Jr, Sever J. L. The role of viruses as causes of congenital diseases. Annu Rev Microbiol. 1971;25:255–282. doi: 10.1146/annurev.mi.25.100171.001351. [DOI] [PubMed] [Google Scholar]
- Coid C. R., Ramsden D. B. Retardation of foetal growth and plasma protein development in foetuses from mice injected with Coxsackie B3 virus. Nature. 1973 Feb 16;241(5390):460–461. doi: 10.1038/241460a0. [DOI] [PubMed] [Google Scholar]
- Coid C. R., Wardman G. The effect of maternal respiratory disease induced by para-influenza type 1 (Sendai) virus on foetal development and neonatal mortality in the rat. Med Microbiol Immunol. 1972;157(3):181–185. doi: 10.1007/BF02121160. [DOI] [PubMed] [Google Scholar]
- Coid C. R., Wardman G. The effect of para-influenza type 1 (Sendai) virus infection on early pregnancy in the rat. J Reprod Fertil. 1971 Jan;24(1):39–43. doi: 10.1530/jrf.0.0240039. [DOI] [PubMed] [Google Scholar]
- Coleman T. J., Gamble D. R., Taylor K. W. Diabetes in mice after Coxsackie B 4 virus infection. Br Med J. 1973 Jul 7;3(5870):25–27. doi: 10.1136/bmj.3.5870.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotlier E., Fox J., Bohigian G., Beaty C., Du Pree A. Pathogenic effects of rubella virus on embryos and newborn rats. Nature. 1968 Jan 6;217(5123):38–40. doi: 10.1038/217038a0. [DOI] [PubMed] [Google Scholar]
- DALLDORF G., GIFFORD R. Susceptibility of gravid mice to Coxsackie virus infection. J Exp Med. 1954 Jan 1;99(1):21–27. doi: 10.1084/jem.99.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Edwards M. J. Influenza, hyperthermia, and congenital malformation. Lancet. 1972 Feb 5;1(7745):320–321. doi: 10.1016/s0140-6736(72)90327-3. [DOI] [PubMed] [Google Scholar]
- Enwonwu C. O., Glover V. Effect of maternal malnutrition during pregnancy and lactation on hepatic protein metabolism in the infant rat: biochemical and ultrastructural studies. Am J Clin Nutr. 1973 Jan;26(1):3–16. doi: 10.1093/ajcn/26.1.3. [DOI] [PubMed] [Google Scholar]
- Enwonwu C. O., Sreebny L. M. Studies of hepatic lesions of experimental protein-calorie malnutrition in rats and immediate effects of refeeding an adequate protein diet. J Nutr. 1971 Apr;101(4):501–514. doi: 10.1093/jn/101.4.501. [DOI] [PubMed] [Google Scholar]
- Gomori G. Observations with differential stains on human islets of langerhans. Am J Pathol. 1941 May;17(3):395–406.3. [PMC free article] [PubMed] [Google Scholar]
- Harrison A. K., Bauer S. P., Murphy F. A. Viral pancreatitis: ultrastructural pathological effects of Coxsackievirus B3 infection in newborn mouse pancreas. Exp Mol Pathol. 1972 Oct;17(2):206–219. doi: 10.1016/0014-4800(72)90070-6. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Hiroi Y., Natori Y. Effect of ATP on protein degradation in rat liver lysosomes. Nat New Biol. 1973 Apr 11;242(119):163–166. doi: 10.1038/newbio242163a0. [DOI] [PubMed] [Google Scholar]
- KALTER H., WARKANY J. Experimental production of congenital maiformations in mammals by metabolic procedure. Physiol Rev. 1959 Jan;39(1):69–115. doi: 10.1152/physrev.1959.39.1.69. [DOI] [PubMed] [Google Scholar]
- Mims C. A. Pathogenesis of viral infections of the fetus. Prog Med Virol. 1968;10:194–237. [PubMed] [Google Scholar]
- Overall J. C., Jr Intrauterine virus infections and congenital heart disease. Am Heart J. 1972 Dec;84(6):823–833. doi: 10.1016/0002-8703(72)90077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUPTEL E. A., MAXIMOVICH N. A. THE POSSIBILITY OF INTRAUTERINE INFECTION WITH COXSACKIE VIRUS IN MICE INOCULATED BY DIFFERENT ROUTES. Acta Virol. 1964 Jan;8:46–51. [PubMed] [Google Scholar]
- SWIFT H., HRUBAN Z. FOCAL DEGRADATION AS A BIOLOGICAL PROCESS. Fed Proc. 1964 Sep-Oct;23:1026–1037. [PubMed] [Google Scholar]
- Saxena U. C., Roy S. K. Effect of transitory protein supplements on early fetal development in rats. Indian J Exp Biol. 1972 Jan;10(1):70–72. [PubMed] [Google Scholar]
- Sever J. L. Virus infections and malformations. Fed Proc. 1971 Jan-Feb;30(1):114–117. [PubMed] [Google Scholar]
- Tsui C. Y., Burch G. E., Harb J. M. Pancreatitis in mice infected with coxsackievirus B1. Arch Pathol. 1972 May;93(5):379–389. [PubMed] [Google Scholar]
- Töndury G., Töndury T. A. Uber den Infektionsweg und die Pathogenese von viruserkrankungen des menschlichen Embryo. Rev Suisse Zool. 1972;(Suppl):179–196. [PubMed] [Google Scholar]
- Zeman F. J. Effect of the young rat of maternal protein restriction. J Nutr. 1967 Oct;93(2):167–173. doi: 10.1093/jn/93.2.167. [DOI] [PubMed] [Google Scholar]
- Zeman F. J., Stanbrough E. C. Effect of maternal protein deficiency on cellular development in the fetal rat. J Nutr. 1969 Nov;99(3):274–282. doi: 10.1093/jn/99.3.274. [DOI] [PubMed] [Google Scholar]