Abstract
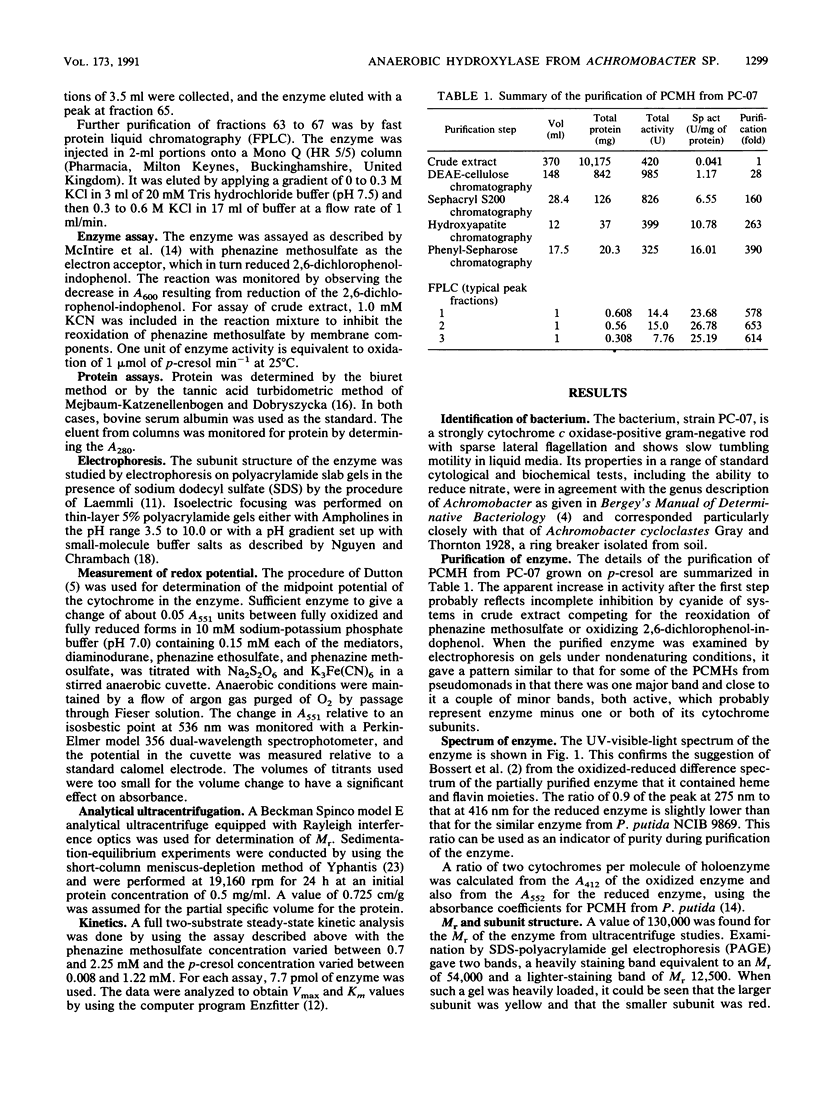
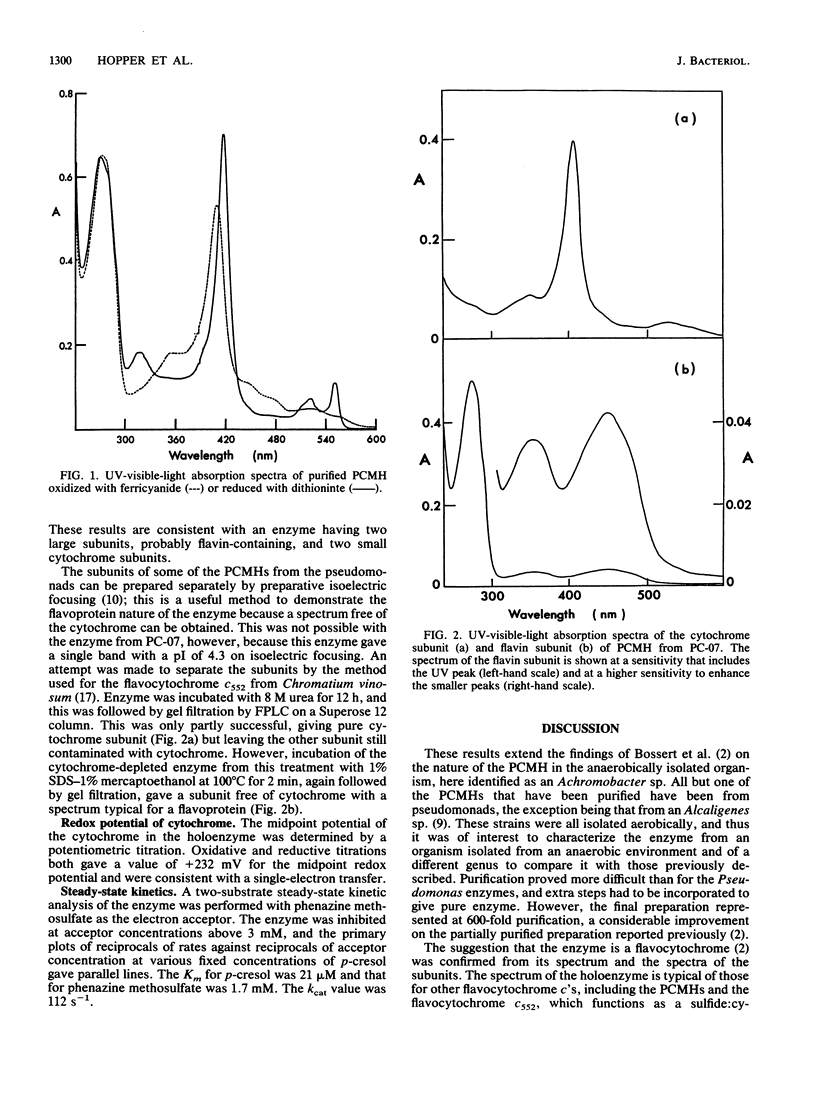
A bacterium, strain PC-07, previously isolated as part of a coculture capable of growing on p-cresol under anaerobic conditions with nitrate as the acceptor was identified as an Achromobacter sp. The first enzyme of the pathway, p-cresol methylhydroxylase, which converts its substrate into p-hydroxybenzyl alcohol, was purified. The enzyme had an Mr of 130,000 and the spectrum of a flavocytochrome. It was composed of flavoprotein subunits of Mr 54,000 and cytochrome subunits of Mr 12,500. The midpoint redox potential of the cytochrome was 232 mV. The Km and kcat for p-cresol were 21 microM and 112 s-1 respectively, and the Km for phenazine methosulfate, the artificial acceptor used in the assays, was determined to be 1.7 mM. These properties place the enzyme in the same class as the p-cresol methylhydroxylases from aerobically isolated Pseudomonas spp.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bossert I. D., Whited G., Gibson D. T., Young L. Y. Anaerobic oxidation of p-cresol mediated by a partially purified methylhydroxylase from a denitrifying bacterium. J Bacteriol. 1989 Jun;171(6):2956–2962. doi: 10.1128/jb.171.6.2956-2962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert I. D., Young L. Y. Anaerobic oxidation of p-cresol by a denitrifying bacterium. Appl Environ Microbiol. 1986 Nov;52(5):1117–1122. doi: 10.1128/aem.52.5.1117-1122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Hopper D. J. Incorporation of [18O]water in the formation of p-hydroxybenzyl alcohol by the p-cresol methylhydroxylase from Pseudomonas putida. Biochem J. 1978 Oct 1;175(1):345–347. doi: 10.1042/bj1750345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J. Redox potential of the cytochrome c in the flavocytochrome p-cresol methylhydroxylase. FEBS Lett. 1983 Sep 5;161(1):100–102. doi: 10.1016/0014-5793(83)80738-8. [DOI] [PubMed] [Google Scholar]
- Hopper D. J. The hydroxylation of P-cresol and its conversion to P-hydroxybenzaldehyde in Pseudomonas putida. Biochem Biophys Res Commun. 1976 Mar 22;69(2):462–468. doi: 10.1016/0006-291x(76)90544-1. [DOI] [PubMed] [Google Scholar]
- Koerber S. C., Hopper D. J., McIntire W. S., Singer T. P. Formation and properties of flavoprotein-cytochrome hybrids by recombination of subunits from different species. Biochem J. 1985 Oct 15;231(2):383–387. doi: 10.1042/bj2310383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McIntire W. S., Hopper D. J., Singer T. P. Steady-state and stopped-flow kinetic measurements of the primary deuterium isotope effect in the reaction catalyzed by p-cresol methylhydroxylase. Biochemistry. 1987 Jun 30;26(13):4107–4117. doi: 10.1021/bi00387a055. [DOI] [PubMed] [Google Scholar]
- McIntire W., Edmondson D. E., Hopper D. J., Singer T. P. 8 alpha-(O-Tyrosyl)flavin adenine dinucleotide, the prosthetic group of bacterial p-cresol methylhydroxylase. Biochemistry. 1981 May 26;20(11):3068–3075. doi: 10.1021/bi00514a013. [DOI] [PubMed] [Google Scholar]
- McIntire W., Hopper D. J., Singer T. P. p-Cresol methylhydroxylase. Assay and general properties. Biochem J. 1985 Jun 1;228(2):325–335. doi: 10.1042/bj2280325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. E., Vorkink W. P., Tollin G., Cusanovich M. A. Chromatium flavocytochrome c: kinetics of reduction of the heme subunit, and the flavocytochrome c-mitochondrial cytochrome c complex. Arch Biochem Biophys. 1985 Jan;236(1):52–58. doi: 10.1016/0003-9861(85)90605-8. [DOI] [PubMed] [Google Scholar]
- Reeve C. D., Carver M. A., Hopper D. J. The purification and characterization of 4-ethylphenol methylenehydroxylase, a flavocytochrome from Pseudomonas putida JD1. Biochem J. 1989 Oct 15;263(2):431–437. doi: 10.1042/bj2630431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolenski W. J., Suflita J. M. Biodegradation of cresol isomers in anoxic aquifers. Appl Environ Microbiol. 1987 Apr;53(4):710–716. doi: 10.1128/aem.53.4.710-716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschech A., Fuchs G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol. 1987 Sep;148(3):213–217. doi: 10.1007/BF00414814. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]



