Abstract
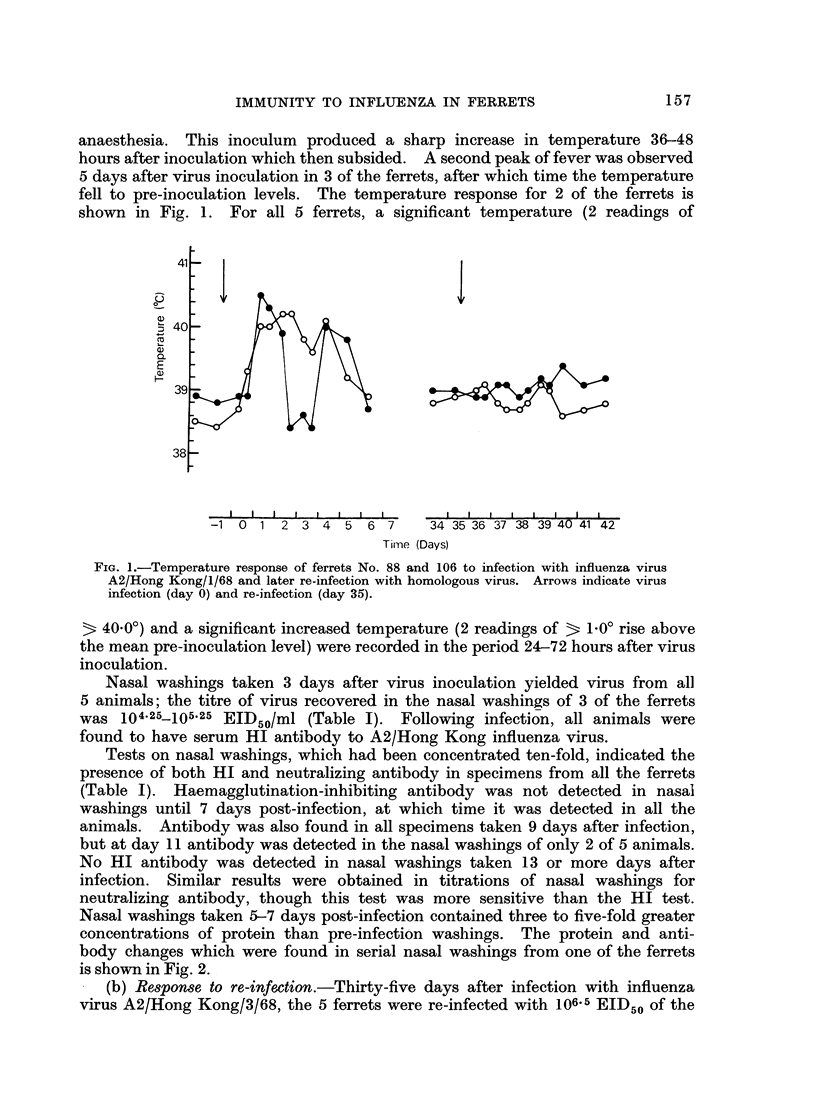
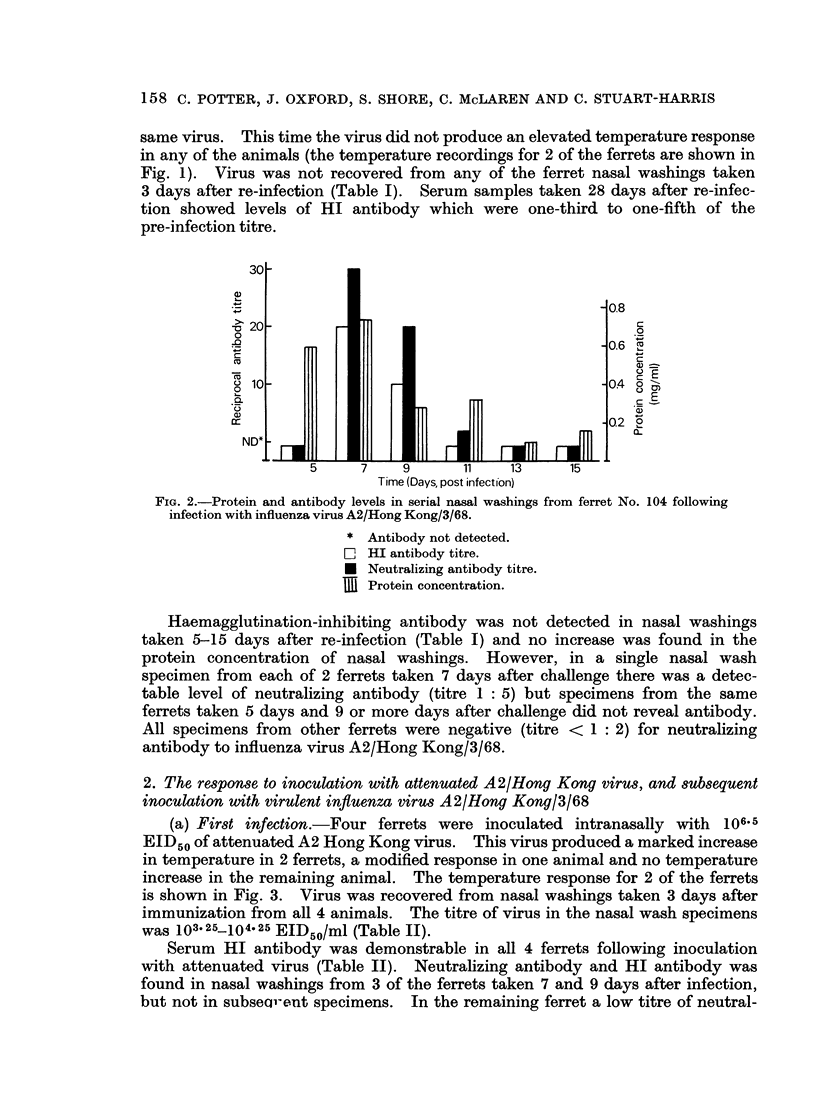
Ferrets were found to react with a sharp febrile response to intranasal infection with influenza virus A2/Hong Kong/3/68. Virus was recovered from nasal washings taken 3 days after infection, and virus antibody was found in serum specimens taken 21 days after virus infection. Virus infection produced a pronounced rhinitis; the protein concentration in nasal washings was found to increase three to five-fold with peak levels occurring on day 7, post-infection. Concomitant with the increased protein levels, detectable levels of HI and neutralizing antibody were found in the nasal washings. However, nasal washings taken 13 days or more after influenza virus infection did not contain either increased levels of protein or detectable antibody. These ferrets were immune to re-infection with homologous virus inoculated 5 weeks after primary infection. Thus, ferrets showed no febrile response; virus was not recovered from nasal washings; serum antibody titres did not increase; no increase in protein levels was found in nasal washings; and HI antibody was not found in nasal washings.
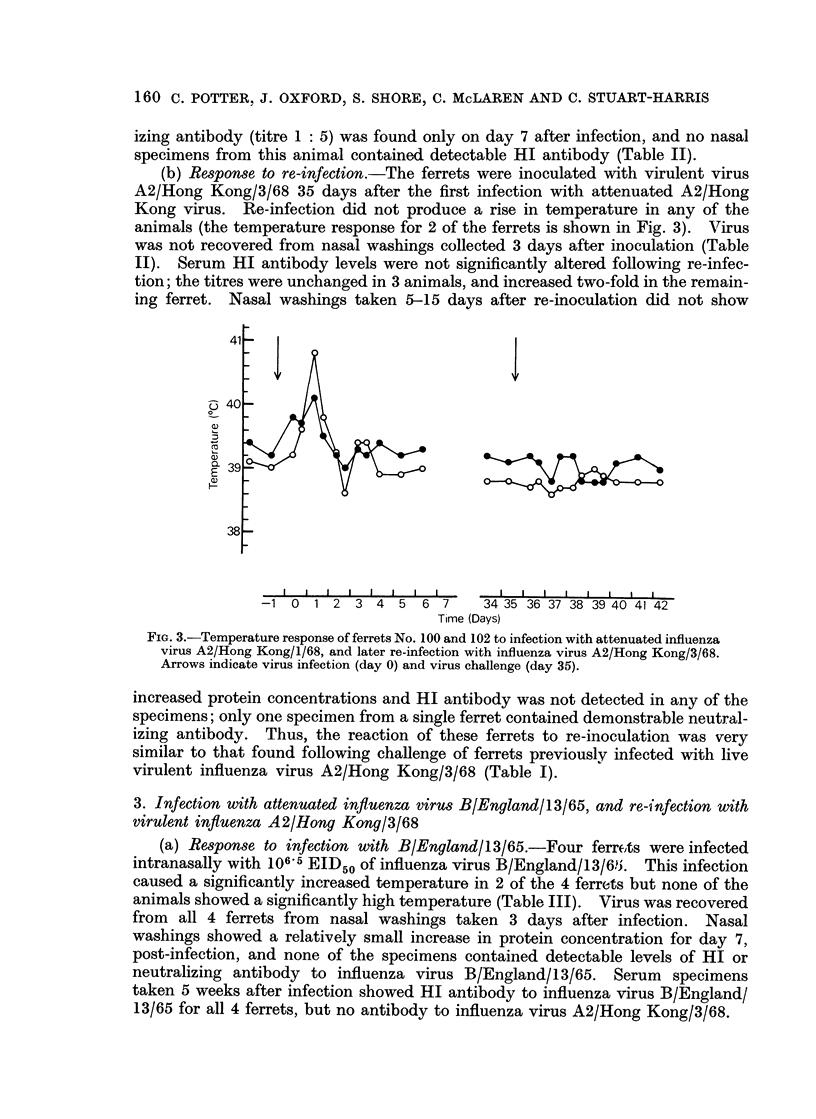
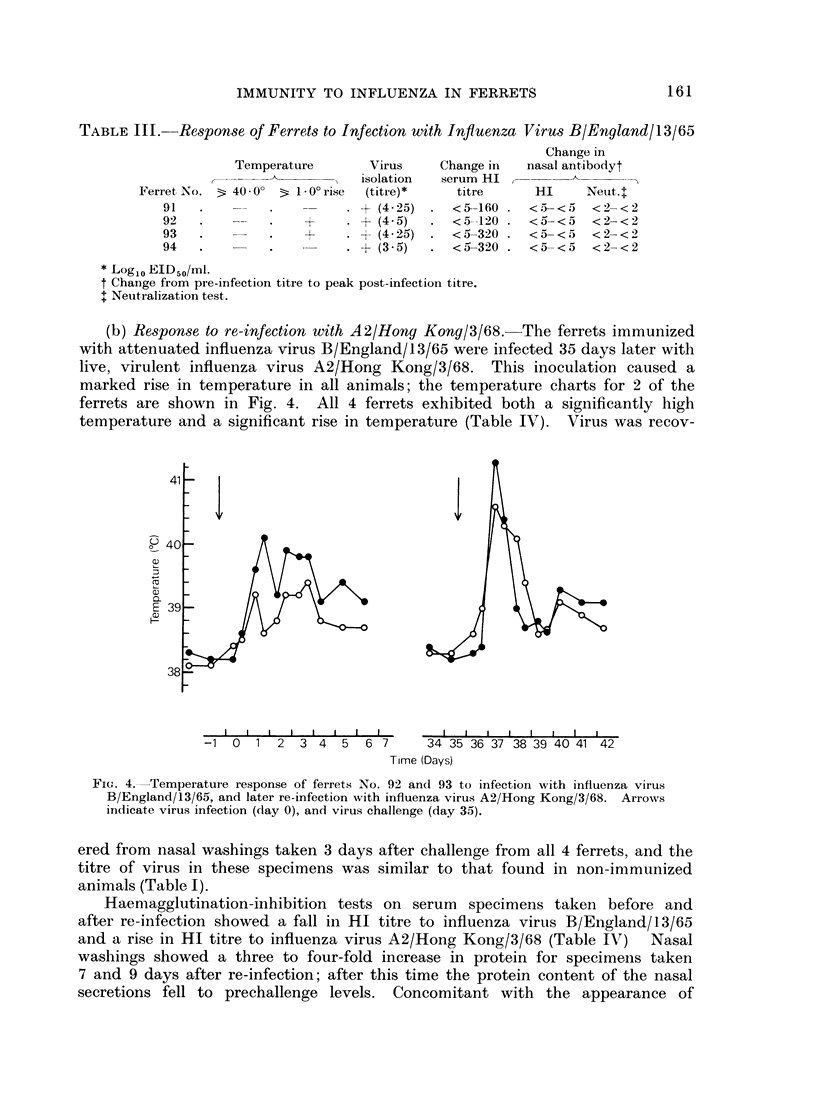
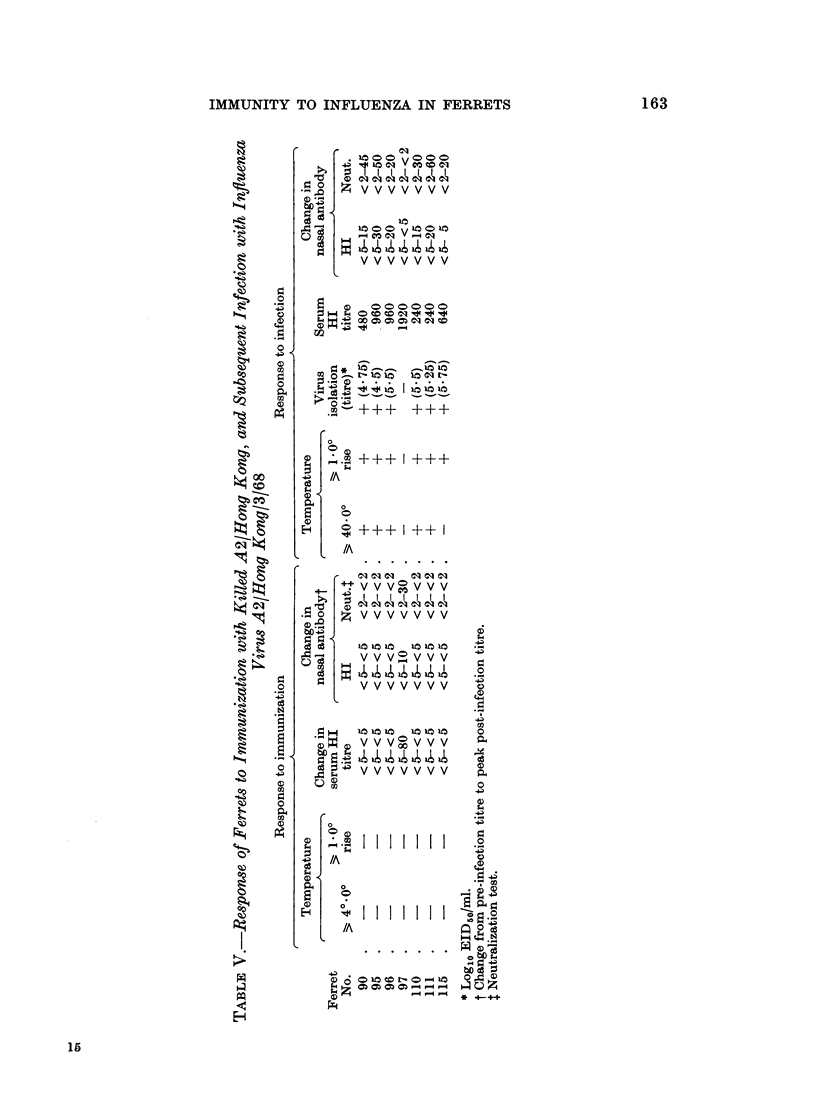
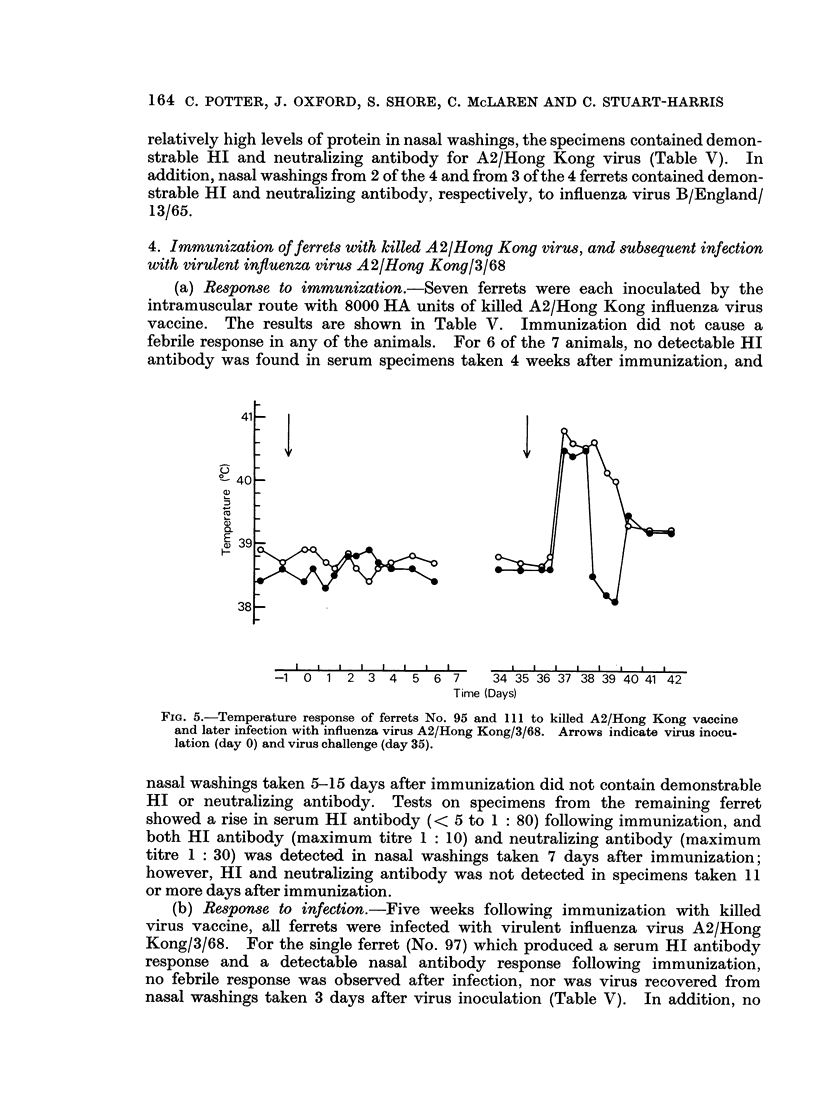
Using these criteria to assess susceptibility or immunity to influenza virus infection, infection with attenuated influenza virus A2/Hong Kong/1/68 produced immunity to re-infection with virulent virus. Ferrets infected with influenza virus B/England/13/65 or immunized with killed A2/Hong Kong virus did not induce any immunity to infection with influenza virus A2/Hong Kong/3/68.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beare A. S., Bynoe M. L., Tyrrell D. A. Investigetion into the attenuation of influenza viruses by serial passage. Br Med J. 1968 Nov 23;4(5629):482–484. doi: 10.1136/bmj.4.5629.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haff R. F., Schriver P. W., Stewart R. C. Pathogenesis of influenza in ferrets: nasal manifestations of disease. Br J Exp Pathol. 1966 Oct;47(5):435–444. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Machin S. J., Potter C. W., Oxford J. S. Changes in the antibody status of a population following epidemic infection by influenza virus A2-Hong Kong-1-68. J Hyg (Lond) 1970 Sep;68(3):497–504. doi: 10.1017/s0022172400042406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois P., Boudreault A., DiFranco E., Pavilanis V. Response of ferrets and monkeys to intranasal infection with human, equine and avian influenza viruses. Can J Comp Med. 1971 Jan;35(1):71–76. [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Shore S. L., McLaren C., Stuart-Harris C. Immunity to influenza in ferrets. II. Influence of adjuvants on immunization. Br J Exp Pathol. 1972 Apr;53(2):168–179. [PMC free article] [PubMed] [Google Scholar]
- SUGG J. Y., NAGAKI D. Tissue tropism of a strain of influenza A virus in ferrets. J Immunol. 1955 Jan;74(1):46–50. [PubMed] [Google Scholar]


