Abstract
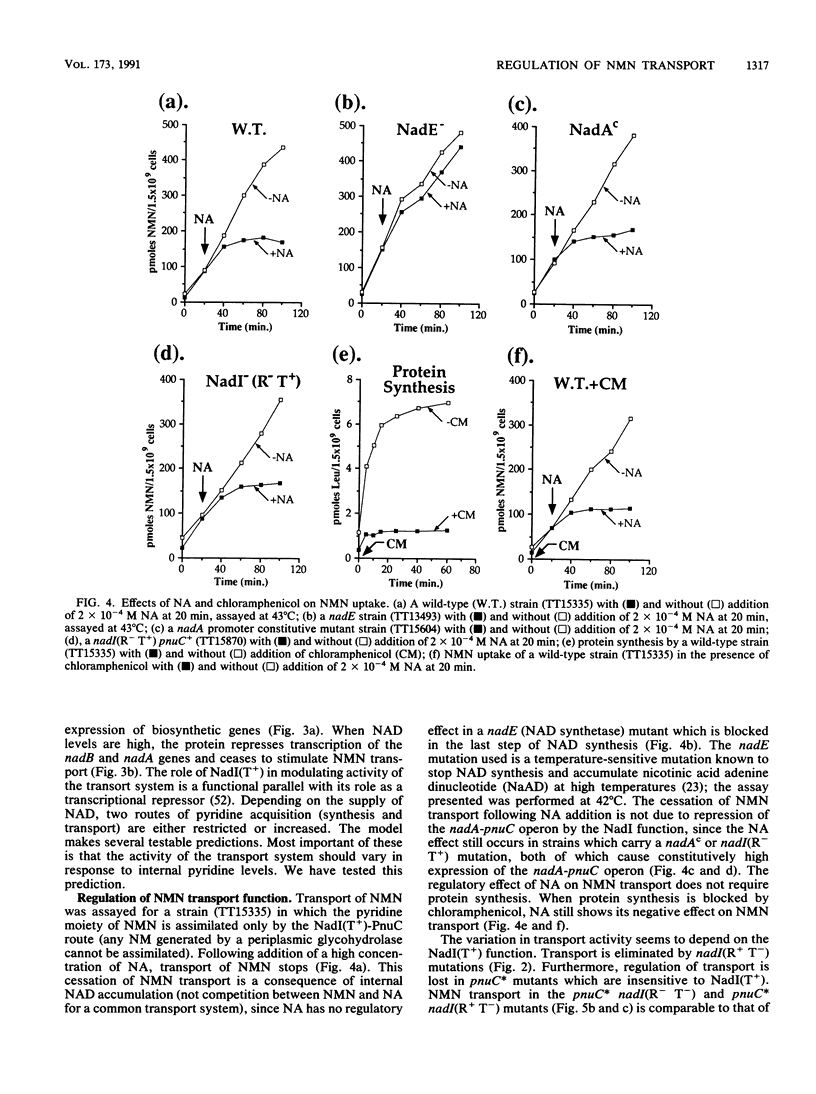
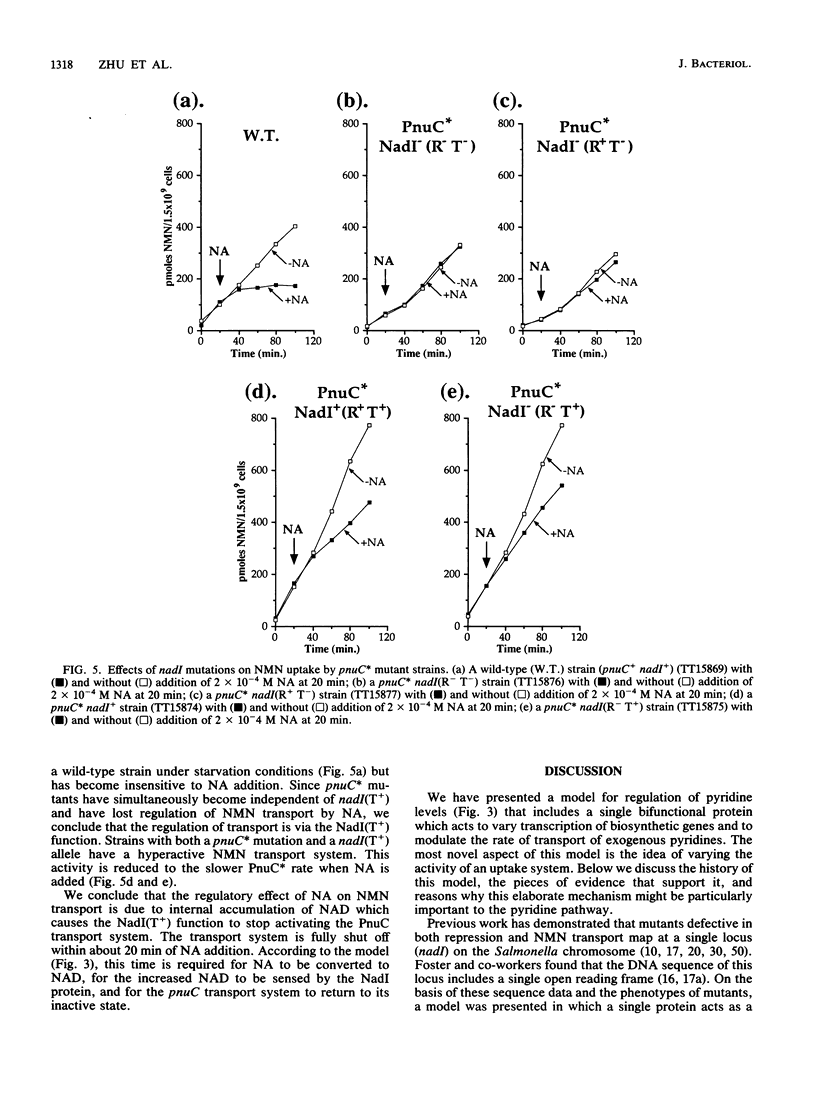
Transport of nicotinamide mononucleotide (NMN) requires two functions, NadI(T) and PnuC. The PnuC protein is membrane associated, as judged by isolation of active TnphoA gene fusions and demonstration that the fusion protein is membrane associated. The PnuC function appears to be the major component of the transport system, since mutant alleles of the pnuC gene permit NMN transport in the absence of NadI(T) function. We present evidence that the activity of the NMN transport system varies in response to internal pyridine levels (presumably NAD). This control mechanism requires NadI(T) function, which is provided by a bifunctional protein encoded by the nadI gene (called nadR by Foster and co-workers [J. W. Foster, Y. K. Park, T. Fenger, and M. P. Spector, J. Bacteriol. 172:4187-4196]). The nadI protein regulates transcription of the nadA and nadB biosynthetic genes and modulates activity of the NMN permease; both regulatory activities respond to the internal pyridine nucleotide level.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ito K. Topology analysis of the SecY protein, an integral membrane protein involved in protein export in Escherichia coli. EMBO J. 1987 Nov;6(11):3465–3470. doi: 10.1002/j.1460-2075.1987.tb02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli A. J., Okita T. W., Bloom R., Grover T. A. The pyridine nucleotide cycle: presence of a nicotinamide mononucleotide-specific glycohydrolase in Escherichia coli. Biochem Biophys Res Commun. 1972 Oct 6;49(1):264–269. doi: 10.1016/0006-291x(72)90039-3. [DOI] [PubMed] [Google Scholar]
- Boyd D., Manoil C., Beckwith J. Determinants of membrane protein topology. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8525–8529. doi: 10.1073/pnas.84.23.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Botstein D., Watanabe T., Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972 Dec;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S. Y., Parkinson J. S. Bacterial motility: membrane topology of the Escherichia coli MotB protein. Science. 1988 Jan 15;239(4837):276–278. doi: 10.1126/science.2447650. [DOI] [PubMed] [Google Scholar]
- Cookson B. T., Olivera B. M., Roth J. R. Genetic characterization and regulation of the nadB locus of Salmonella typhimurium. J Bacteriol. 1987 Sep;169(9):4285–4293. doi: 10.1128/jb.169.9.4285-4293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Kapoor M. Purification and some properties of the glutamate dehydrogenase of Salmonella typhimurium. Can J Microbiol. 1973 Apr;19(4):427–438. doi: 10.1139/m73-071. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Roth J. R. Characterization of Tn10d-Cam: a transposition-defective Tn10 specifying chloramphenicol resistance. Mol Gen Genet. 1988 Aug;213(2-3):332–338. doi: 10.1007/BF00339599. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Holley-Guthrie E. A., Warren F. Regulation of NAD metabolism in Salmonella typhimurium: genetic analysis and cloning of the nadR repressor locus. Mol Gen Genet. 1987 Jun;208(1-2):279–287. doi: 10.1007/BF00330454. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Kinney D. M., Moat A. G. Pyridine nucleotide cycle of Salmonella typhimurium: isolation and characterization of pncA, pncB, and pncC mutants and utilization of exogenous nicotinamide adenine dinucleotide. J Bacteriol. 1979 Mar;137(3):1165–1175. doi: 10.1128/jb.137.3.1165-1175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W. Pyridine nucleotide cycle of Salmonella typhimurium: in vitro demonstration of nicotinamide adenine dinucleotide glycohydrolase, nicotinamide mononucleotide glycohydrolase, and nicotinamide adenine dinucleotide pyrophosphatase activities. J Bacteriol. 1981 Feb;145(2):1002–1009. doi: 10.1128/jb.145.2.1002-1009.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard D., Rechsteiner M., Manlapaz-Ramos P., Imperial J. S., Cruz L. J., Olivera B. M. The pyridine nucleotide cycle. Studies in Escherichia coli and the human cell line D98/AH2. J Biol Chem. 1981 Aug 25;256(16):8491–8497. [PubMed] [Google Scholar]
- Hoffman C. S., Wright A. Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5107–5111. doi: 10.1073/pnas.82.15.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley E. A., Spector M. P., Foster J. W. Regulation of NAD biosynthesis in Salmonella typhimurium: expression of nad-lac gene fusions and identification of a nad regulatory locus. J Gen Microbiol. 1985 Oct;131(10):2759–2770. doi: 10.1099/00221287-131-10-2759. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Ladika D., Roth J. R., Olivera B. M. An indispensable gene for NAD biosynthesis in Salmonella typhimurium. J Bacteriol. 1983 Jul;155(1):213–221. doi: 10.1128/jb.155.1.213-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Olivera B. M., Roth J. R. Structural gene for NAD synthetase in Salmonella typhimurium. J Bacteriol. 1988 May;170(5):2113–2120. doi: 10.1128/jb.170.5.2113-2120.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988 May;119(1):9–12. doi: 10.1093/genetics/119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Stock J. B., Silhavy T. J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989 Nov;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Jaworowski A., Campbell H. D., Poulis M. I., Young I. G. Genetic identification and purification of the respiratory NADH dehydrogenase of Escherichia coli. Biochemistry. 1981 Mar 31;20(7):2041–2047. doi: 10.1021/bi00510a047. [DOI] [PubMed] [Google Scholar]
- Kier L. D., Weppelman R. M., Ames B. N. Regulation of nonspecific acid phosphatase in Salmonella: phoN and phoP genes. J Bacteriol. 1979 Apr;138(1):155–161. doi: 10.1128/jb.138.1.155-161.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney D. M., Foster J. W., Moat A. G. Pyridine nucleotide cycle of Salmonella typhimurium: in vitro demonstration of nicotinamide mononucleotide deamidase and characterization of pnuA mutants defective in nicotinamide mononucleotide transport. J Bacteriol. 1979 Nov;140(2):607–611. doi: 10.1128/jb.140.2.607-611.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Foster J., Manlapaz-Ramos P., Olivera B. M. Nucleoside salvage pathway for NAD biosynthesis in Salmonella typhimurium. J Bacteriol. 1982 Dec;152(3):1111–1116. doi: 10.1128/jb.152.3.1111-1116.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Hunt J. F., Beckwith J. Effects of signal sequence mutations on the kinetics of alkaline phosphatase export to the periplasm in Escherichia coli. J Bacteriol. 1986 Jul;167(1):160–167. doi: 10.1128/jb.167.1.160-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa A. J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Bonhoeffer F. Discontinuous DNA replication in vitro. I. Two distinct size classes of intermediates. Nat New Biol. 1972 Dec 20;240(103):233–235. doi: 10.1038/newbio240233a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Bonhoeffer F. Replication of Phi-X174 DNA by Escherichia coli polA- in vitro (Phi-X174 DNA-DNA replication-E. coli polA-). Proc Natl Acad Sci U S A. 1972 Jan;69(1):25–29. doi: 10.1073/pnas.69.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Protein phosphorylation in bacterial chemotaxis. Cell. 1988 Apr 8;53(1):1–2. doi: 10.1016/0092-8674(88)90478-3. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Takahashi I., Yamagishi S. Iodometric assay method for beta-lactamase with various beta-lactam antibiotics as substrates. Antimicrob Agents Chemother. 1978 Jun;13(6):910–913. doi: 10.1128/aac.13.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M. B., Roth J. R. Genetic methods for analysis and manipulation of inversion mutations in bacteria. Genetics. 1983 Nov;105(3):517–537. doi: 10.1093/genetics/105.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. A method for detection of phage mutants with altered transducing ability. Mol Gen Genet. 1971;110(4):378–381. doi: 10.1007/BF00438281. [DOI] [PubMed] [Google Scholar]
- Spector M. P., Hill J. M., Holley E. A., Foster J. W. Genetic characterization of pyridine nucleotide uptake mutants of Salmonella typhimurium. J Gen Microbiol. 1985 Jun;131(6):1313–1322. doi: 10.1099/00221287-131-6-1313. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Youderian P., Sugiono P., Brewer K. L., Higgins N. P., Elliott T. Packaging specific segments of the Salmonella chromosome with locked-in Mud-P22 prophages. Genetics. 1988 Apr;118(4):581–592. doi: 10.1093/genetics/118.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Olivera B. M., Roth J. R. Genetic characterization of the pnuC gene, which encodes a component of the nicotinamide mononucleotide transport system in Salmonella typhimurium. J Bacteriol. 1989 Aug;171(8):4402–4409. doi: 10.1128/jb.171.8.4402-4409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Olivera B. M., Roth J. R. Identification of a repressor gene involved in the regulation of NAD de novo biosynthesis in Salmonella typhimurium. J Bacteriol. 1988 Jan;170(1):117–125. doi: 10.1128/jb.170.1.117-125.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Roth J. R. The nadI region of Salmonella typhimurium encodes a bifunctional regulatory protein. J Bacteriol. 1991 Feb;173(3):1302–1310. doi: 10.1128/jb.173.3.1302-1310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]