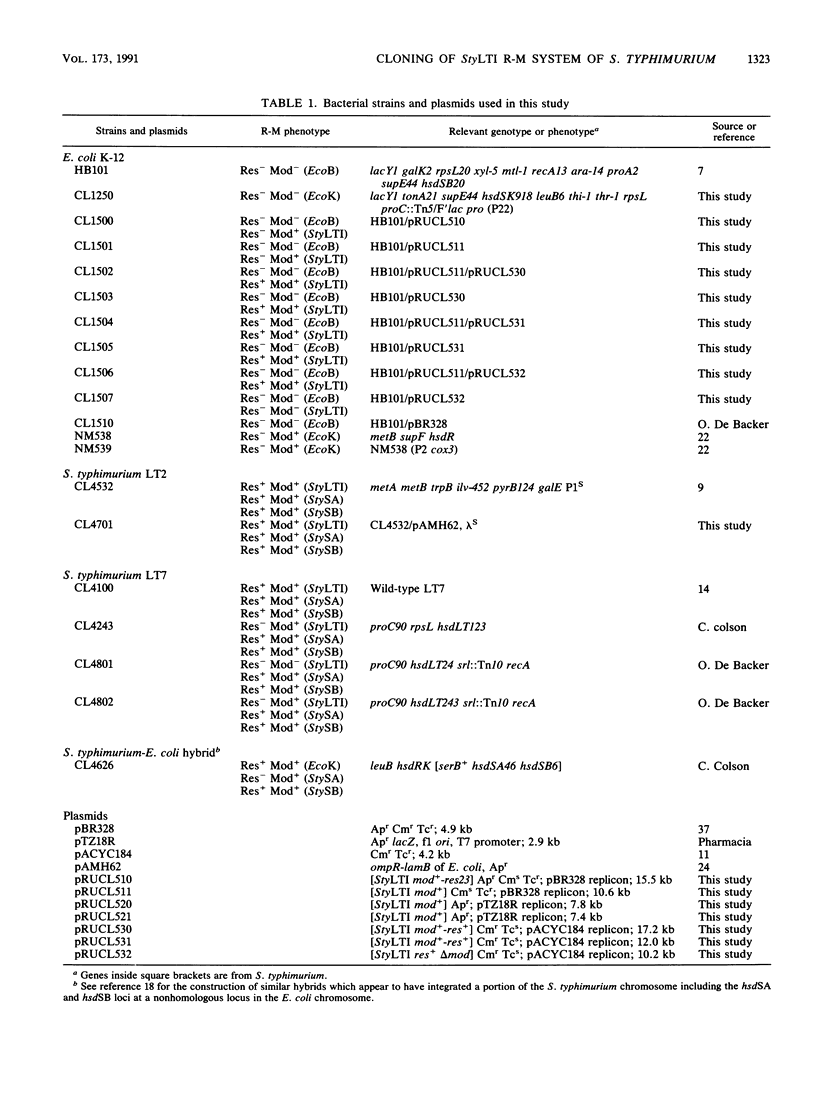
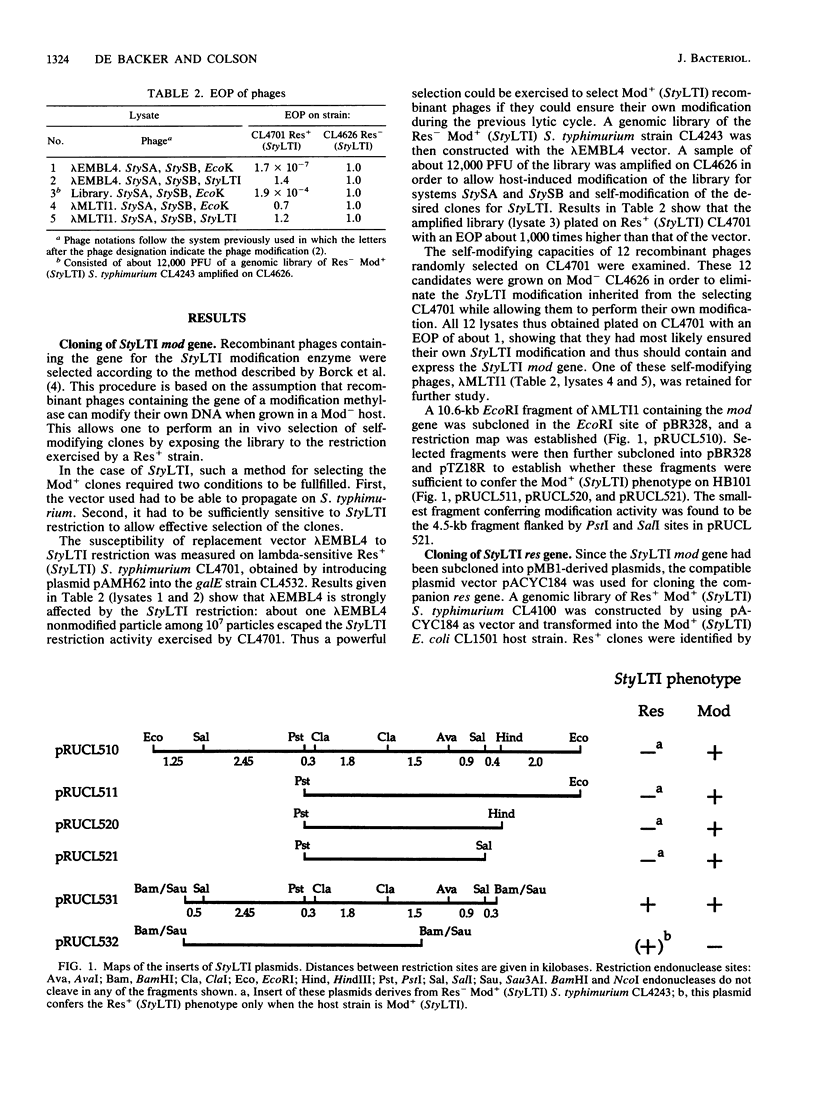
Abstract
The StyLTI restriction-modification system is common to most strains of the genus Salmonella, including Salmonella typhimurium. We report here the two-step cloning of the genes controlling the StyLTI system. The StyLTI methylase gene (mod) was cloned first. Then, the companion endonuclease gene (res) was introduced on a compatible vector. A strain of S. typhimurium sensitive to the coliphage lambda was constructed and used to select self-modifying recombinant phages from a Res- Mod+ S. typhimurium genomic library in the lambda EMBL4 cloning vector. The methylase gene of one of these phages was then subcloned in pBR328 and transferred into Escherichia coli. In the second step, the closely linked endonuclease and methylase genes were cloned together on a single DNA fragment inserted in pACYC184 and introduced into the Mod+ E. coli strain obtained in the first step. Attempts to transform Mod- E. coli or S. typhimurium strains with this Res+ Mod+ plasmid were unsuccessful, whereas transformation of Mod+ strains occurred at a normal frequency. This can be understood if the introduction of the StyLTI genes into naive hosts is lethal because of degradation of host DNA by restriction activity; in contrast to most restriction-modification systems, StyLTI could not be transferred into naive hosts without killing them. In addition, it was found that strains containing only the res gene are viable and lack restriction activity in the absence of the companion mod gene. This suggests that expression of the StyLTI endonuclease activity requires at least one polypeptide involved in the methylation activity, as is the case for types I and III restriction-modification systems but not for type II systems.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., DUSSOIX D. Host specificity of DNA produced by Escherichia coli. I. Host controlled modification of bacteriophage lambda. J Mol Biol. 1962 Jul;5:18–36. doi: 10.1016/s0022-2836(62)80058-8. [DOI] [PubMed] [Google Scholar]
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Botstein D., Herskowitz I. Properties of hybrids between Salmonella phage P22 and coliphage lambda. Nature. 1974 Oct 18;251(5476):584–589. doi: 10.1038/251584a0. [DOI] [PubMed] [Google Scholar]
- Bougueleret L., Schwarzstein M., Tsugita A., Zabeau M. Characterization of the genes coding for the Eco RV restriction and modification system of Escherichia coli. Nucleic Acids Res. 1984 Apr 25;12(8):3659–3676. doi: 10.1093/nar/12.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Colson C. DNA restriction and modification systems in Salmonella. III. SP, a Salmonella potsdam system allelic to the SB system in Salmonella typhimurium. Mol Gen Genet. 1975 Aug 27;139(3):177–188. [PubMed] [Google Scholar]
- Bullas L. R., Colson C., Neufeld B. Deoxyribonucleic acid restriction and modification systems in Salmonella: chromosomally located systems of different serotypes. J Bacteriol. 1980 Jan;141(1):275–292. doi: 10.1128/jb.141.1.275-292.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson A. M., Colson C. Expression of the Escherichia coli K, B and phage P1 DNA host specificities in Salmonella typhimurium. J Gen Microbiol. 1972 Apr;70(1):123–128. doi: 10.1099/00221287-70-1-123. [DOI] [PubMed] [Google Scholar]
- Colson A. M., Colson C., Van Pel A. Host-controlled restriction mutants of Salmonella typhimurium. J Gen Microbiol. 1969 Sep;58(1):57–64. doi: 10.1099/00221287-58-1-57. [DOI] [PubMed] [Google Scholar]
- Colson C., Colson A. M. A new Salmonella typhimurium DNA host specificity. J Gen Microbiol. 1971 Dec;69(3):345–351. doi: 10.1099/00221287-69-3-345. [DOI] [PubMed] [Google Scholar]
- Colson C., Colson A. M. Host specificity and fertility in Salmonella typhimurium LT7. Biochem Biophys Res Commun. 1967 Dec 15;29(5):692–695. doi: 10.1016/0006-291x(67)90272-0. [DOI] [PubMed] [Google Scholar]
- Colson C., Colson A. M., Van Pel A. Chromosomal location of host specificity in Salmonella typhimurium. J Gen Microbiol. 1970 Feb;60(2):265–271. doi: 10.1099/00221287-60-2-265. [DOI] [PubMed] [Google Scholar]
- Colson C., Glover S. W., Symonds N., Stacey K. A. The location of the genes for host-controlled modification and restriction in Escherichia coli K-12. Genetics. 1965 Nov;52(5):1043–1050. doi: 10.1093/genetics/52.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson C., Van Pel A. DNA restriction and modification systems in Salmonella. I. SA and SB, two Salmonella typhimurium systems determined by genes with a chromosomal location comparable to that of the Escherichia coli hsd genes. Mol Gen Genet. 1974 Apr 3;129(4):325–337. doi: 10.1007/BF00265696. [DOI] [PubMed] [Google Scholar]
- De Backer O., Colson C. Transfer of the genes for the StyLTI restriction-modification system of Salmonella typhimurium to strains lacking modification ability results in death of the recipient cells and degradation of their DNA. J Bacteriol. 1991 Feb;173(3):1328–1330. doi: 10.1128/jb.173.3.1328-1330.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R. Evidence that a nucleotide sequence, "boxA," is involved in the action of the NusA protein. Cell. 1983 Aug;34(1):143–149. doi: 10.1016/0092-8674(83)90144-7. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Harkki A., Palva E. T. Application of phage lambda technology to Salmonella typhimurium. Construction of a lambda-sensitive Salmonella strain. Mol Gen Genet. 1984;195(1-2):256–259. doi: 10.1007/BF00332756. [DOI] [PubMed] [Google Scholar]
- Howard K. A., Card C., Benner J. S., Callahan H. L., Maunus R., Silber K., Wilson G., Brooks J. E. Cloning the DdeI restriction-modification system using a two-step method. Nucleic Acids Res. 1986 Oct 24;14(20):7939–7951. doi: 10.1093/nar/14.20.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S., Meyer J., Bächi B., Stålhammar-Carlemalm M., Schrickel S., Bickle T. A., Arber W. DNA restriction--modification genes of phage P1 and plasmid p15B. Structure and in vitro transcription. J Mol Biol. 1983 Mar 25;165(1):1–18. doi: 10.1016/s0022-2836(83)80239-3. [DOI] [PubMed] [Google Scholar]
- Iida S., Streiff M. B., Bickle T. A., Arber W. Two DNA antirestriction systems of bacteriophage P1, darA, and darB: characterization of darA- phages. Virology. 1987 Mar;157(1):156–166. doi: 10.1016/0042-6822(87)90324-2. [DOI] [PubMed] [Google Scholar]
- Looney M. C., Moran L. S., Jack W. E., Feehery G. R., Benner J. S., Slatko B. E., Wilson G. G. Nucleotide sequence of the FokI restriction-modification system: separate strand-specificity domains in the methyltransferase. Gene. 1989 Aug 15;80(2):193–208. doi: 10.1016/0378-1119(89)90284-9. [DOI] [PubMed] [Google Scholar]
- Lunnen K. D., Barsomian J. M., Camp R. R., Card C. O., Chen S. Z., Croft R., Looney M. C., Meda M. M., Moran L. S., Nwankwo D. O. Cloning type-II restriction and modification genes. Gene. 1988 Dec 25;74(1):25–32. doi: 10.1016/0378-1119(88)90242-9. [DOI] [PubMed] [Google Scholar]
- Mural R. J., Chesney R. H., Vapnek D., Kropf M. M., Scott J. R. Isolation and characterization of cloned fragments of bacteriophage P1 DNA. Virology. 1979 Mar;93(2):387–397. doi: 10.1016/0042-6822(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Le Minor L. Occurrence of the bacteriophage lambda receptor in some enterobacteriaceae. J Virol. 1975 Apr;15(4):679–685. doi: 10.1128/jvi.15.4.679-685.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatko B. E., Benner J. S., Jager-Quinton T., Moran L. S., Simcox T. G., Van Cott E. M., Wilson G. G. Cloning, sequencing and expression of the Taq I restriction-modification system. Nucleic Acids Res. 1987 Dec 10;15(23):9781–9796. doi: 10.1093/nar/15.23.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Nathans D. Letter: A suggested nomenclature for bacterial host modification and restriction systems and their enzymes. J Mol Biol. 1973 Dec 15;81(3):419–423. doi: 10.1016/0022-2836(73)90152-6. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Theriault G., Roy P. H., Howard K. A., Benner J. S., Brooks J. E., Waters A. F., Gingeras T. R. Nucleotide sequence of the PaeR7 restriction/modification system and partial characterization of its protein products. Nucleic Acids Res. 1985 Dec 9;13(23):8441–8461. doi: 10.1093/nar/13.23.8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pel A., Colson C. DNA restriction and modification systems in Salmonella. II. Genetic complementation between the K and B systems of Escherichia coli and the Salmonella typhimurium system SB, with the same chromosomal location. Mol Gen Genet. 1974;135(1):51–60. doi: 10.1007/BF00433901. [DOI] [PubMed] [Google Scholar]
- Wilson G. G. Type II restriction--modification systems. Trends Genet. 1988 Nov;4(11):314–318. doi: 10.1016/0168-9525(88)90109-6. [DOI] [PubMed] [Google Scholar]



