Abstract
A quantitative analysis has been made of tracheal gland size and of histochemical changes occurring in goblet cells of the respiratory epithelium, in rats exposed either to tobacco smoke alone or to tobacco smoke with phenylmethyloxadiazole (PMO).
Exposure to tobacco smoke alone causes an increase in goblet cell number with a shift from the production of neutral to acid glycoprotein, mainly sialidase resistant sialomucin but some sialidase sensitive sialomucin and sulphomucin. Acid glycoprotein, and each of its types, appears first at the cell apex. The addition of PMO to the tobacco protects against the increase in goblet cell number but gives no protection against the shift from neutral to acid glycoprotein and causes a larger secretory mass within the goblet cell.
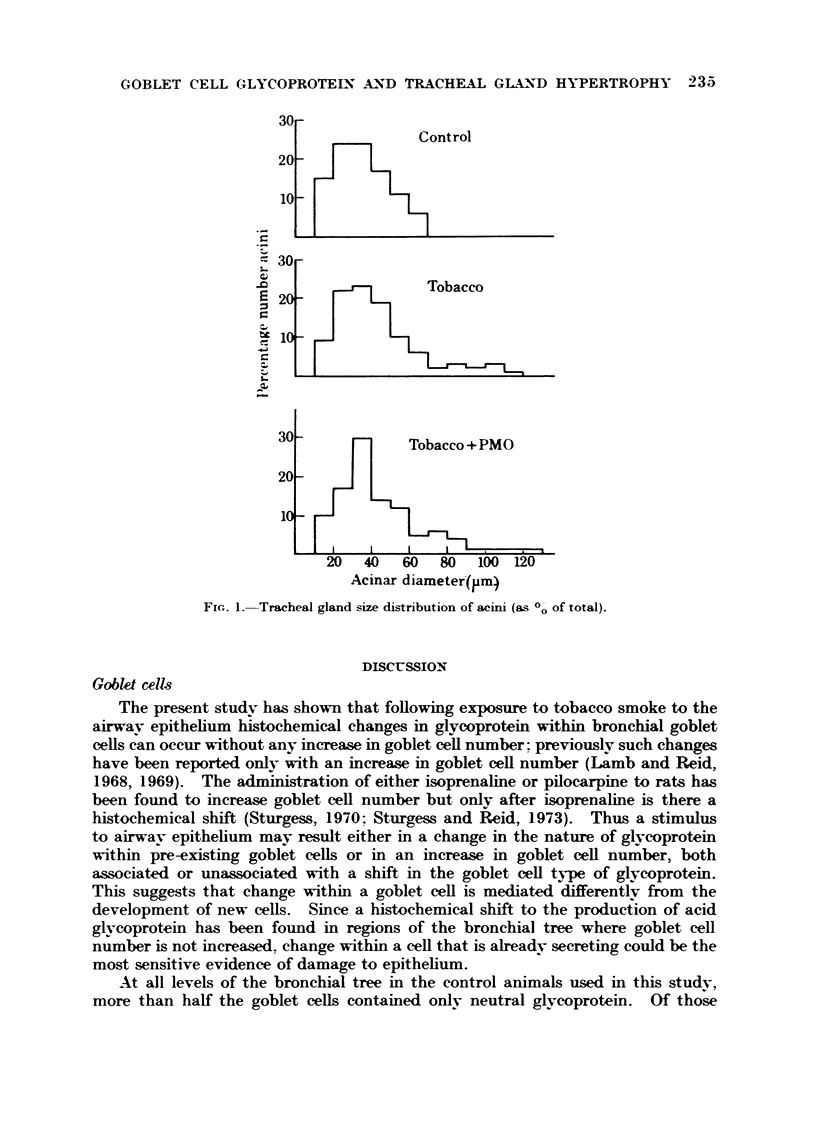
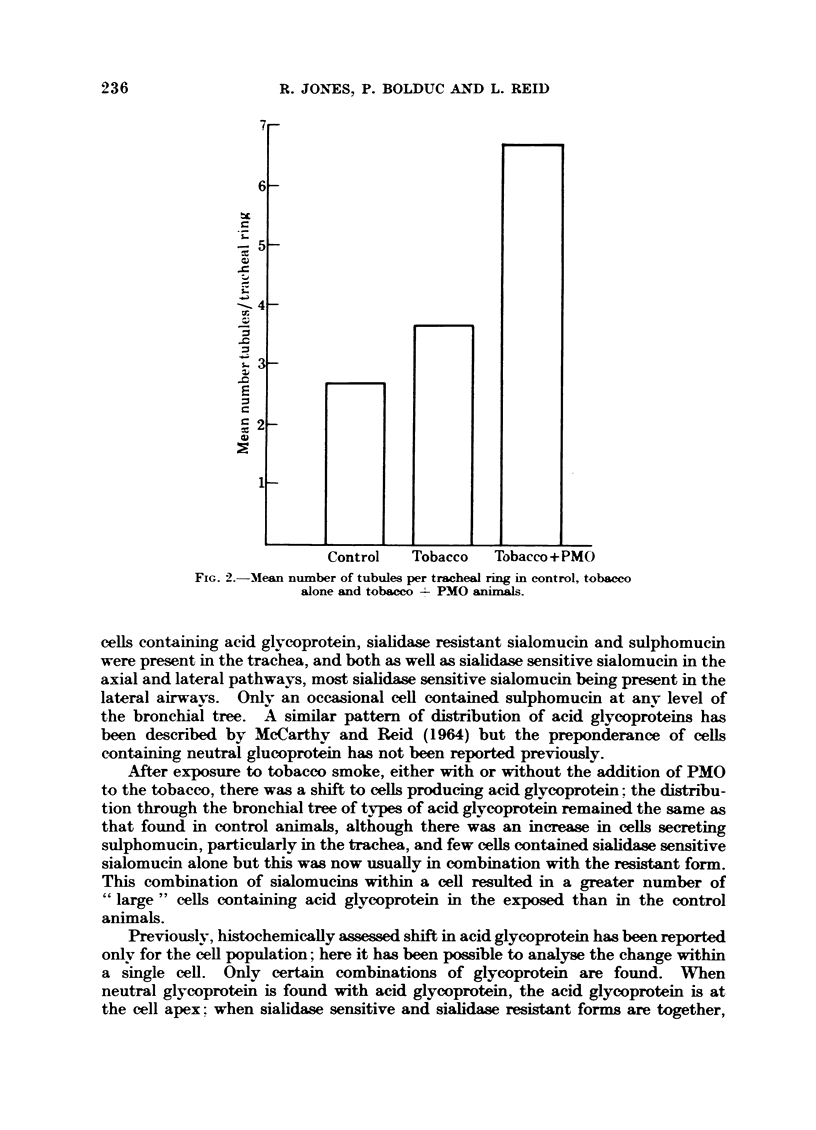
In the tracheal gland exposure to smoke from either tobacco alone or tobacco with PMO causes a significant increase in cell size and acinar diameter and a lesser increase in lumen diameter. There is also an increase in the thickness of the gland and its depth. Each of these gland changes is more pronounced in those animals receiving PMO with the tobacco.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catanese B., Lisciani R., Piccinelli D. Erythrocyte membrane stabilization and protein binding of some anti-inflammatory drugs and of deoxycholic acid. Biochem Pharmacol. 1969 Jul;18(7):1707–1710. doi: 10.1016/0006-2952(69)90160-9. [DOI] [PubMed] [Google Scholar]
- Dalhamn T., Rylander R. Reduction of cigarette smoke ciliotoxicity by certain tobacco additives. Am Rev Respir Dis. 1971 Jun;103(6):855–857. doi: 10.1164/arrd.1971.103.6.855. [DOI] [PubMed] [Google Scholar]
- ELSON L. A., PASSEY R. D. BIOCHEMICAL EFFECTS OF TOBACCO SMOKE AND NICOTINE INHALATION. Acta Unio Int Contra Cancrum. 1963;19:715–717. [PubMed] [Google Scholar]
- Jones R., Bolduc P., Reid L. Protection of rat bronchial epithelium against tobacco smoke. Br Med J. 1972 Apr 15;2(5806):142–144. doi: 10.1136/bmj.2.5806.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANE N., CARO L., OTERO VILARDEBO L. R., GODMAN G. C. ON THE SITE OF SULFATION IN COLONIC GOBLET CELLS. J Cell Biol. 1964 Jun;21:339–351. doi: 10.1083/jcb.21.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima Y., Sakai S., Yamaura M. Mode of stabilizing action of non-steroid anti-inflammatory drugs on erythrocyte membrane. Biochem Pharmacol. 1970 Jan;19(1):227–234. doi: 10.1016/0006-2952(70)90343-6. [DOI] [PubMed] [Google Scholar]
- Neutra M., Leblond C. P. Radioautographic comparison of the uptake of galactose-H and glucose-H3 in the golgi region of various cells secreting glycoproteins or mucopolysaccharides. J Cell Biol. 1966 Jul;30(1):137–150. doi: 10.1083/jcb.30.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whur P., Herscovics A., Leblond C. P. Radioautographic visualization of the incorporation of galactose-3H and mannose-3H by rat thyroids in vitro in relation to the stages of thyroglobulin synthesis. J Cell Biol. 1969 Nov;43(2):289–311. doi: 10.1083/jcb.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. M. Cigarette smoking machine for animal experiments. Lab Pract. 1972 Dec;21(12):881–passim. [PubMed] [Google Scholar]


