Abstract
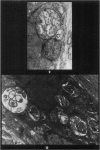
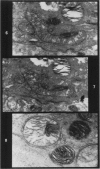
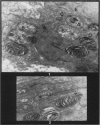
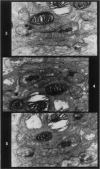
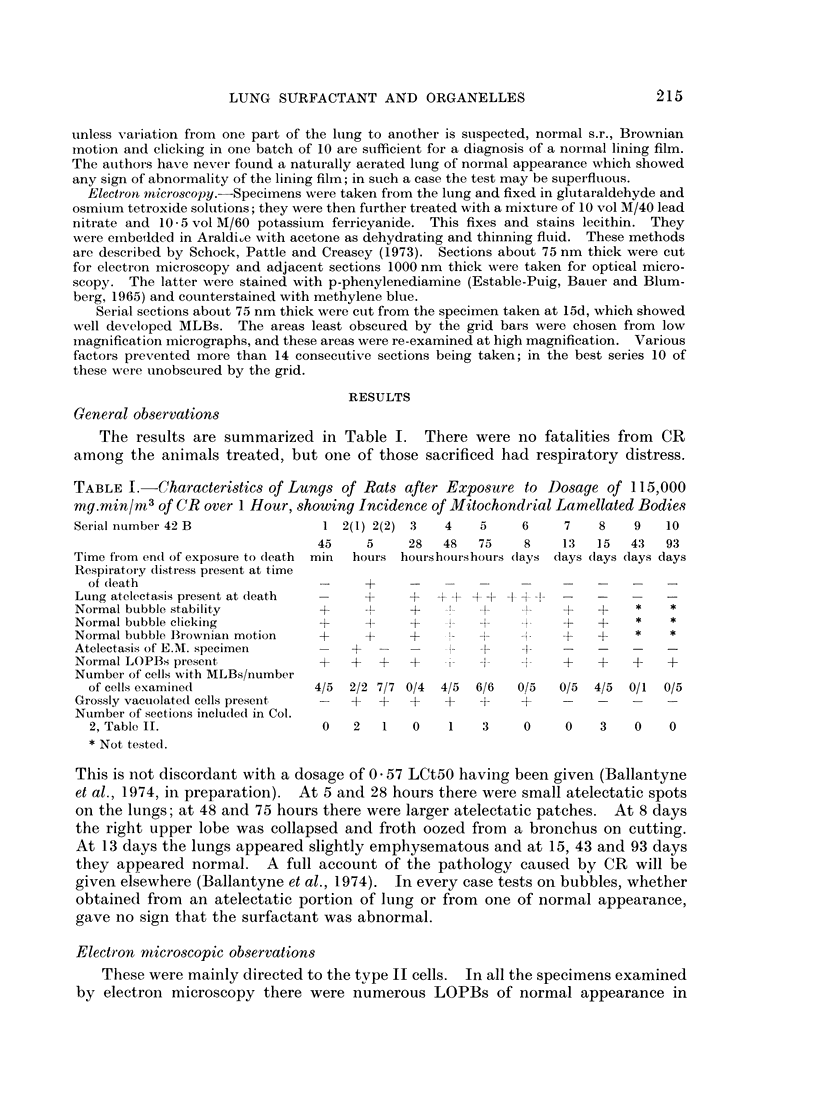
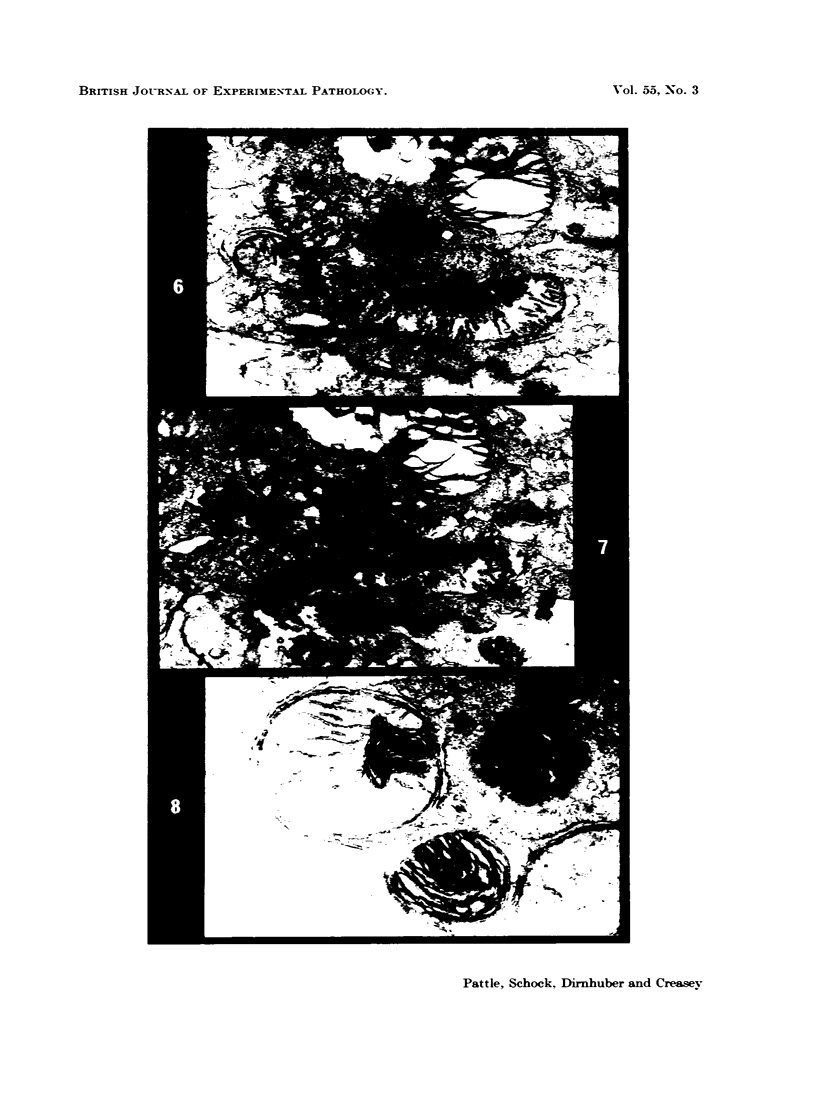
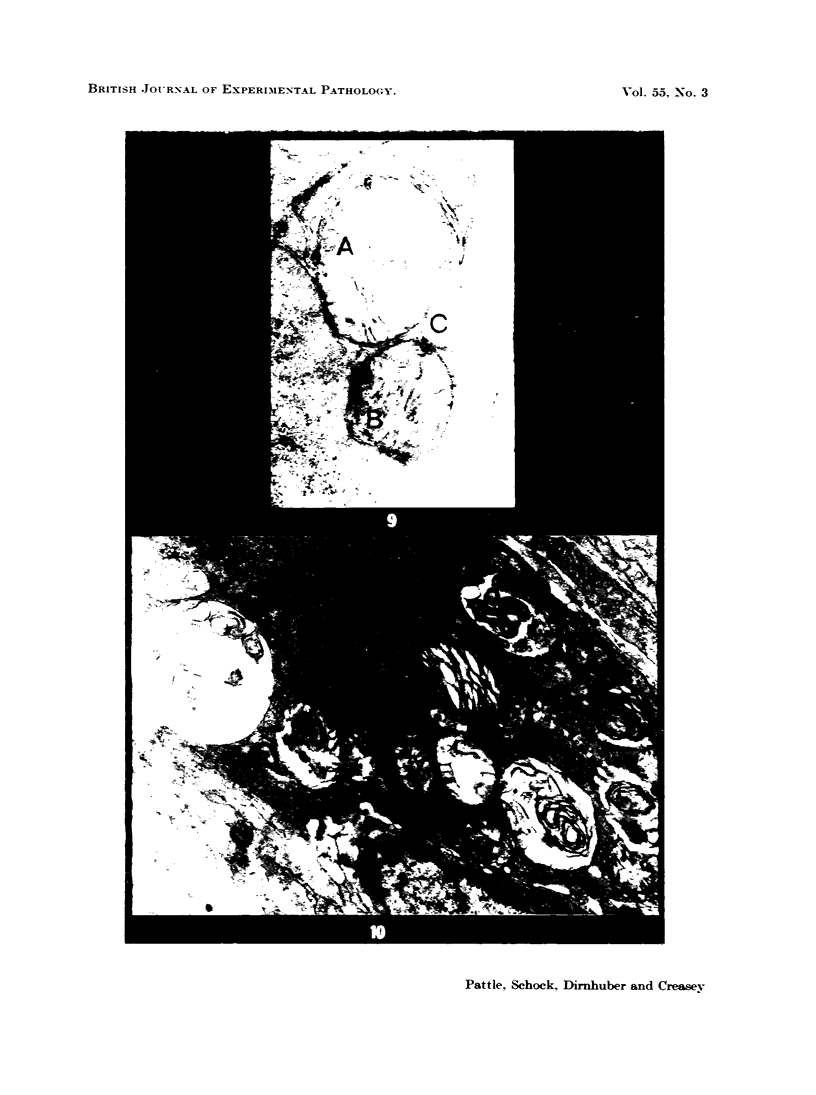
Rats were exposed to a heavy dosage of the sensory irritant dibenz (b.f.)-1,4 oxazepine (CR). No change in the lung surfactant could be detected by the methods used. Electron micrography showed that the ordinary lamellated osmiophilic bodies (LOPBs) and their precursors were unaffected. Bodies containing both mitochondrial cristae and dense osmiophilic whorls (“mitochondrial lamellated bodies”, or MLBs) were found in the type II cells of some animals up to 15 days after the exposure. These whorls originate from the bounding membranes and cristae; serial sections show that they usually abut on the boundary of the organelle. A large proportion of the mitochondria in any cell may be affected by this process. Unequivocal evidence that the MLBs finally evolve into LOPBs without cristae was not obtained in this series; the ultimate fate of the MLBs and the cells containing them is uncertain. The MLBs may perhaps act as an emergency source of surfactant.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Ballantyne B., Beswick F. W., Thomas D. P. The presentation and management of individuals contaminated with solutions of dibenzoxazepine (CR). Med Sci Law. 1973 Oct;13(4):265–268. doi: 10.1177/002580247301300409. [DOI] [PubMed] [Google Scholar]
- Benzer H., Baum M., Lempert J., Tölle W. Bisolvon -Wirkung auf oberflächenaktive Substanzen in der menschlichen Lunge. Dtsch Med J. 1969 May 27;20(10):352–355. [PubMed] [Google Scholar]
- Candiollo L., Filogamo G. Lamellar bodies within the neuroblasts of the neural tube in the chick embryo. Z Zellforsch Mikrosk Anat. 1966;69:480–488. doi: 10.1007/BF00406298. [DOI] [PubMed] [Google Scholar]
- Creasey J. M., Pattle R. E., Schock C. Ultrastructure of inclusion bodies in type II cells of lung, human and sub-simian. J Physiol. 1974 Mar;237(2):35P–37P. [PubMed] [Google Scholar]
- Gil J., Reiss O. K. Isolation and characterization of lamellar bodies and tubular myelin from rat lung homogenates. J Cell Biol. 1973 Jul;58(1):152–171. doi: 10.1083/jcb.58.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. Electron microscopic aspects of surfactant secretion. Proc R Soc Med. 1973 Apr;66(4):386–387. [PMC free article] [PubMed] [Google Scholar]
- PATTLE R. E., BURGESS F. The lung lining film in some pathological conditions. J Pathol Bacteriol. 1961 Oct;82:315–331. [PubMed] [Google Scholar]
- PATTLE R. E., CLAIREAUX A. E., DAVIES P. A., CAMERON A. H. Inability to form a lung-lining film as a cause of the respiratory-distress syndrome in the newborn. Lancet. 1962 Sep 8;2(7254):469–473. doi: 10.1016/s0140-6736(62)90337-9. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. SURFACE LINING OF LUNG ALVEOLI. Physiol Rev. 1965 Jan;45:48–79. doi: 10.1152/physrev.1965.45.1.48. [DOI] [PubMed] [Google Scholar]
- Pannese E. Structures possibly related to the formation of new mitochondria in spinal ganglion neuroblasts. J Ultrastruct Res. 1966 Apr;15(1):57–65. doi: 10.1016/s0022-5320(66)80093-x. [DOI] [PubMed] [Google Scholar]
- Pattle R. E., Schock C., Dirnhuber P., Creasey J. M. Lamellar transformation of lung mitochondria under conditions of stress. Nature. 1972 Dec 22;240(5382):468–469. doi: 10.1038/240468a0. [DOI] [PubMed] [Google Scholar]
- Sacktor B., Shimada Y. Degenerative changes in the mitochondria of flight muscle from aging blowflies. J Cell Biol. 1972 Feb;52(2):465–477. doi: 10.1083/jcb.52.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock C., Pattle R. E., Creasey J. M. Methods for electron microscopy of the lamellated osmiophilic bodies of the lung. J Microsc. 1973 Apr;97(3):321–330. doi: 10.1111/j.1365-2818.1973.tb03787.x. [DOI] [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Spiro A. J., Shy G. M., Gonatas N. K. Myotubular myopathy. Persistence of fetal muscle in an adolescent boy. Arch Neurol. 1966 Jan;14(1):1–14. doi: 10.1001/archneur.1966.00470070005001. [DOI] [PubMed] [Google Scholar]