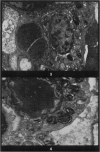
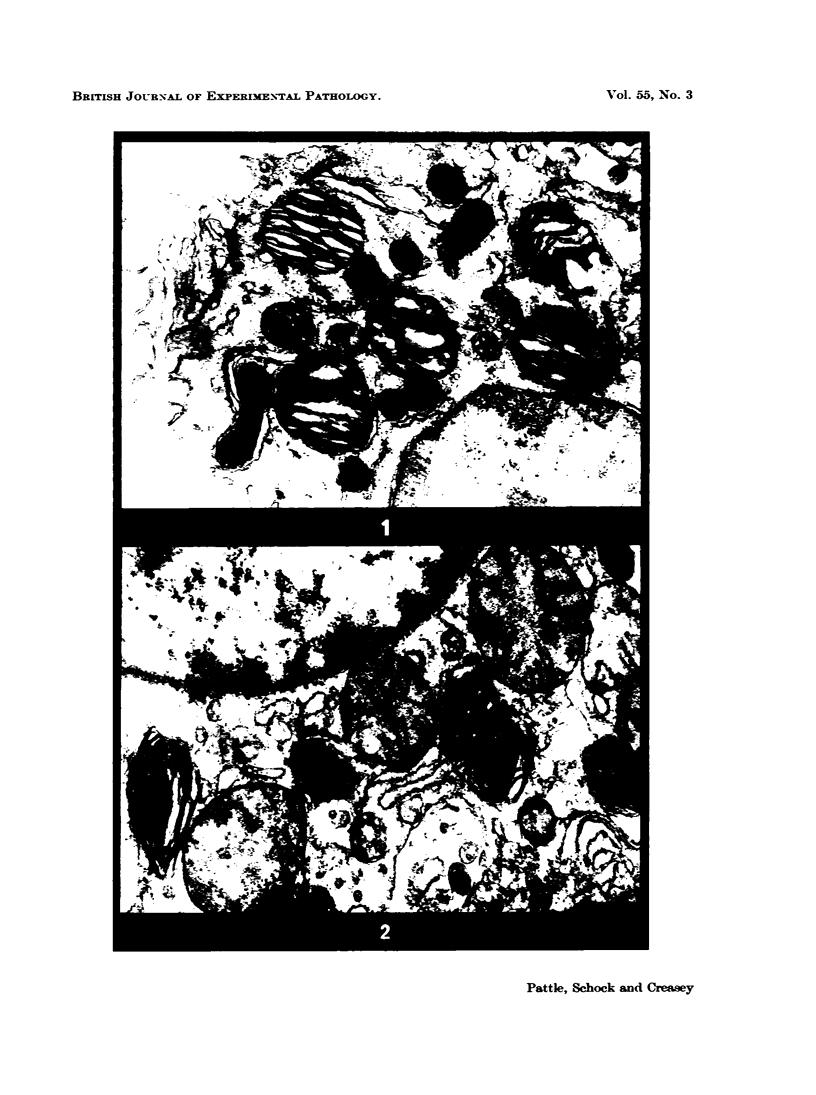
Abstract
In the type II cells of the lungs of mice, left in the cadaver after death, no post-mortem changes at electron microscope level have been found after 30 minutes. At 1 hour cytoplasmic vesicles have appeared and the microvilli have altered. Vesiculation and nuclear contrast then increase, and after 8 hours at 25° most of the cell is occupied by large vesicles and the cell boundary is disrupted. Meanwhile nuclei, mitochondria and lamellated osmiophilic bodies (LOPBs) remain intact. The timing of the changes is irregular but in these cells they are less rapid than the literature (on other cells) would suggest. Useful information about mitochondria and LOPBs can be obtained even after some hours' delay in fixation. The changes seem to be more rapid if the tissues are immersed in saline than if they are left in the cadaver.
Full text
PDF



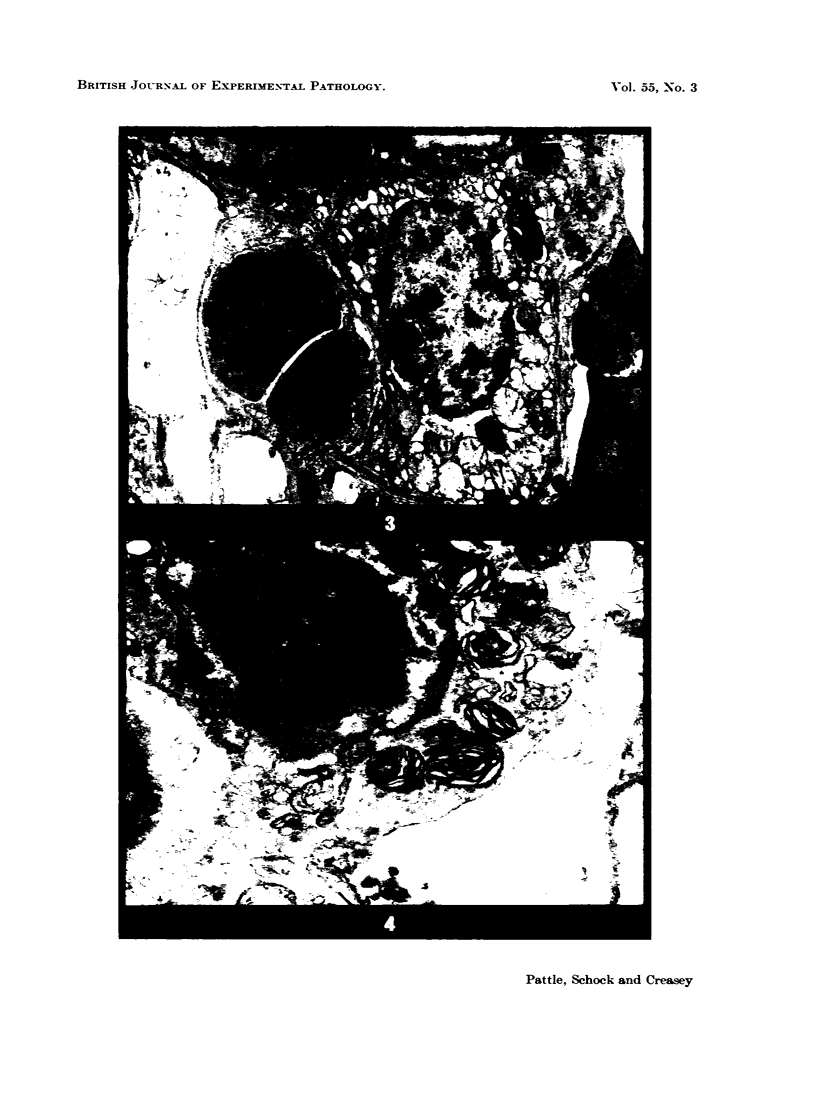



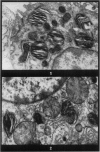
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Creasey J. M., Pattle R. E., Schock C. Ultrastructure of inclusion bodies in type II cells of lung, human and sub-simian. J Physiol. 1974 Mar;237(2):35P–37P. [PubMed] [Google Scholar]
- Gil J., Reiss O. K. Isolation and characterization of lamellar bodies and tubular myelin from rat lung homogenates. J Cell Biol. 1973 Jul;58(1):152–171. doi: 10.1083/jcb.58.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppleston A. G., Young A. E. Alveolar lipo-proteinosis: an ultrastructural comparison of the experimental and human forms. J Pathol. 1972 Jun;107(2):107–117. doi: 10.1002/path.1711070205. [DOI] [PubMed] [Google Scholar]
- MAUNSBACH A. B., MADDEN S. C., LATTA H. Variations in fine structure of renal tubular epithelium under different conditions of fixation. J Ultrastruct Res. 1962 Jun;6:511–530. doi: 10.1016/s0022-5320(62)80006-9. [DOI] [PubMed] [Google Scholar]
- Pattle R. E., Schock C., Dirnhuber P., Creasey J. M. Lamellar transformation of lung mitochondria under conditions of stress. Nature. 1972 Dec 22;240(5382):468–469. doi: 10.1038/240468a0. [DOI] [PubMed] [Google Scholar]
- Pattle R. E., Schock C., Dirnhuber P., Creasey J. M. Lung surfactant and organelles after an exposure to dibenzoxazepine (CR). Br J Exp Pathol. 1974 Jun;55(3):213–220. [PMC free article] [PubMed] [Google Scholar]
- SJOSTRAND F. S., HANZON V. Membrane structures of cytoplasm and mitochondria in exocrine cells of mouse pancreas as revealed by high resolution electron microscopy. Exp Cell Res. 1954 Nov;7(2):393–414. doi: 10.1016/s0014-4827(54)80086-3. [DOI] [PubMed] [Google Scholar]
- Schock C., Pattle R. E., Creasey J. M. Methods for electron microscopy of the lamellated osmiophilic bodies of the lung. J Microsc. 1973 Apr;97(3):321–330. doi: 10.1111/j.1365-2818.1973.tb03787.x. [DOI] [PubMed] [Google Scholar]