Abstract
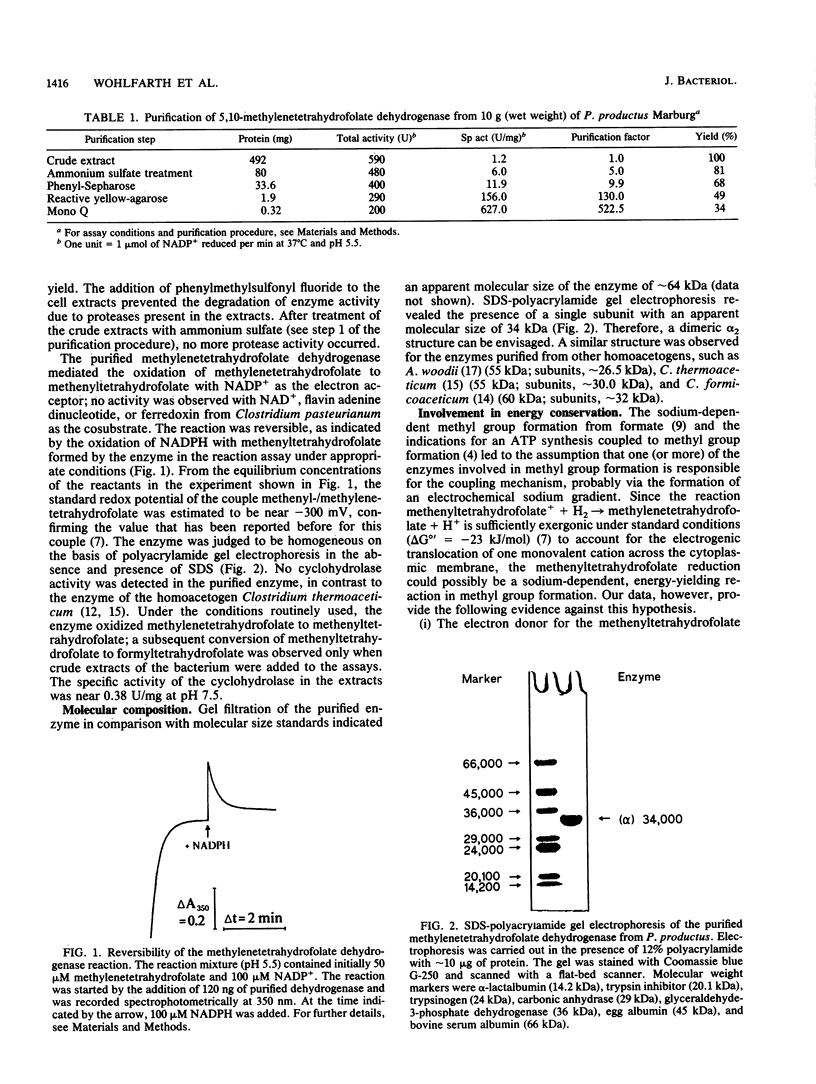
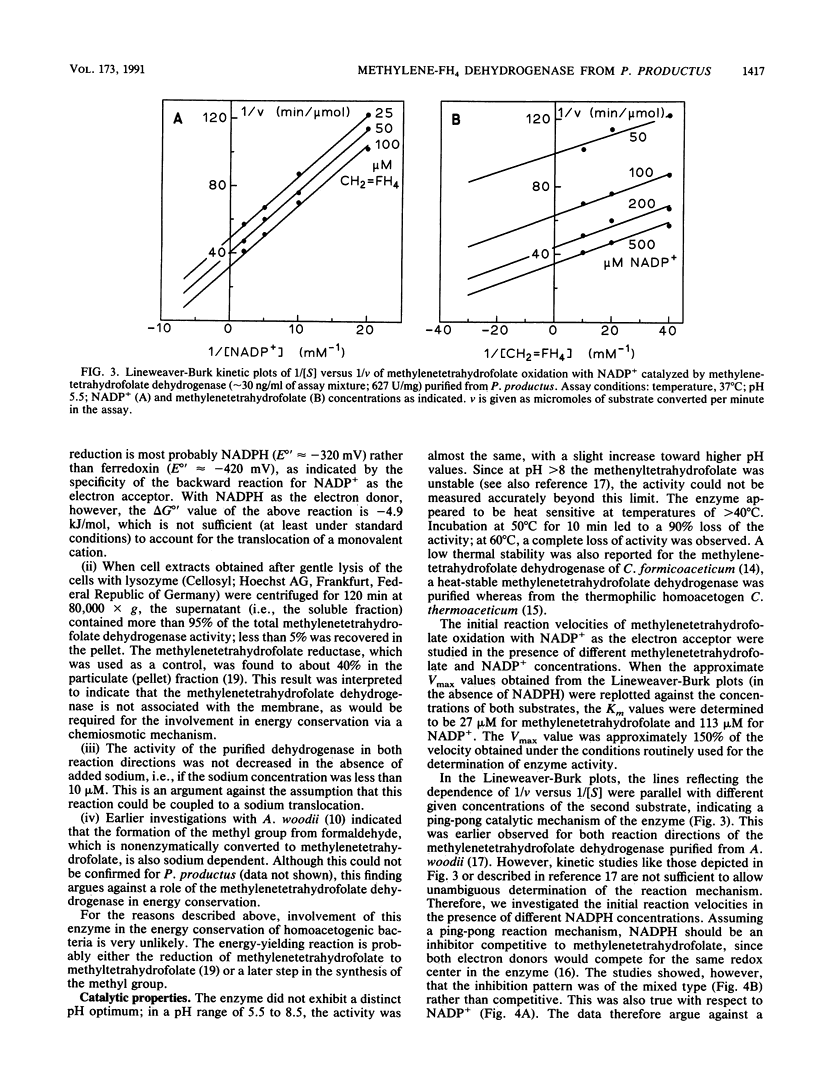
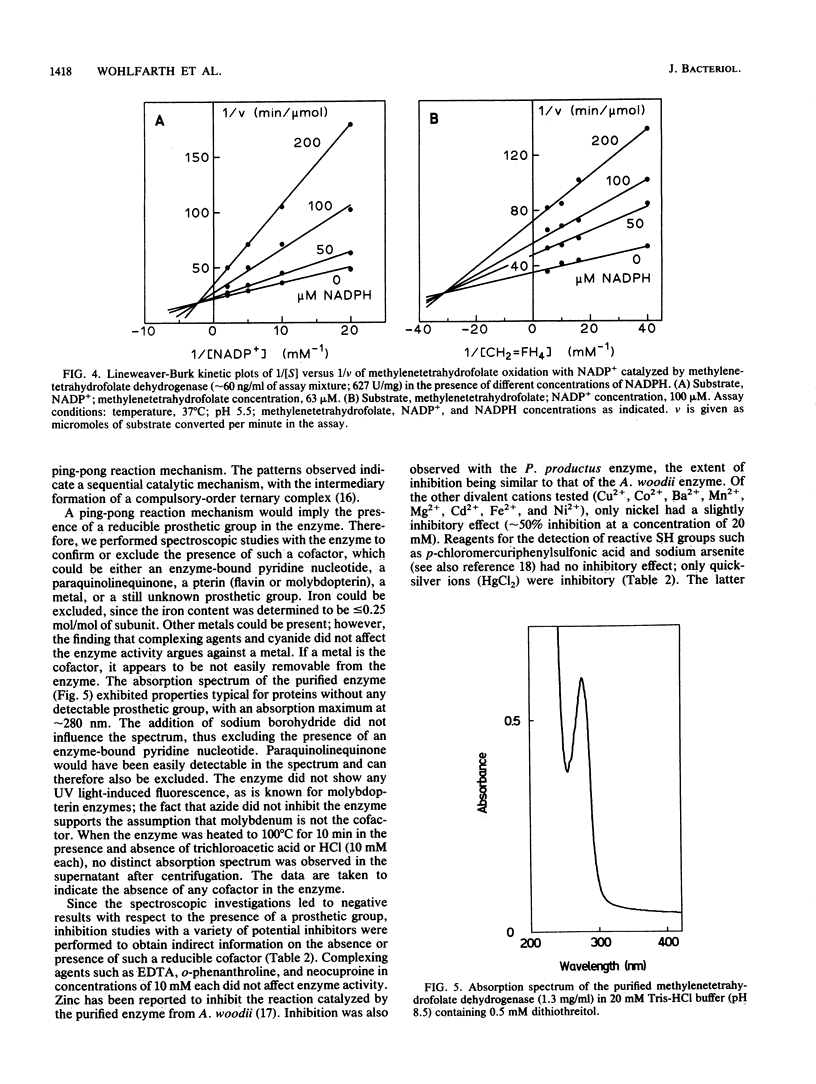
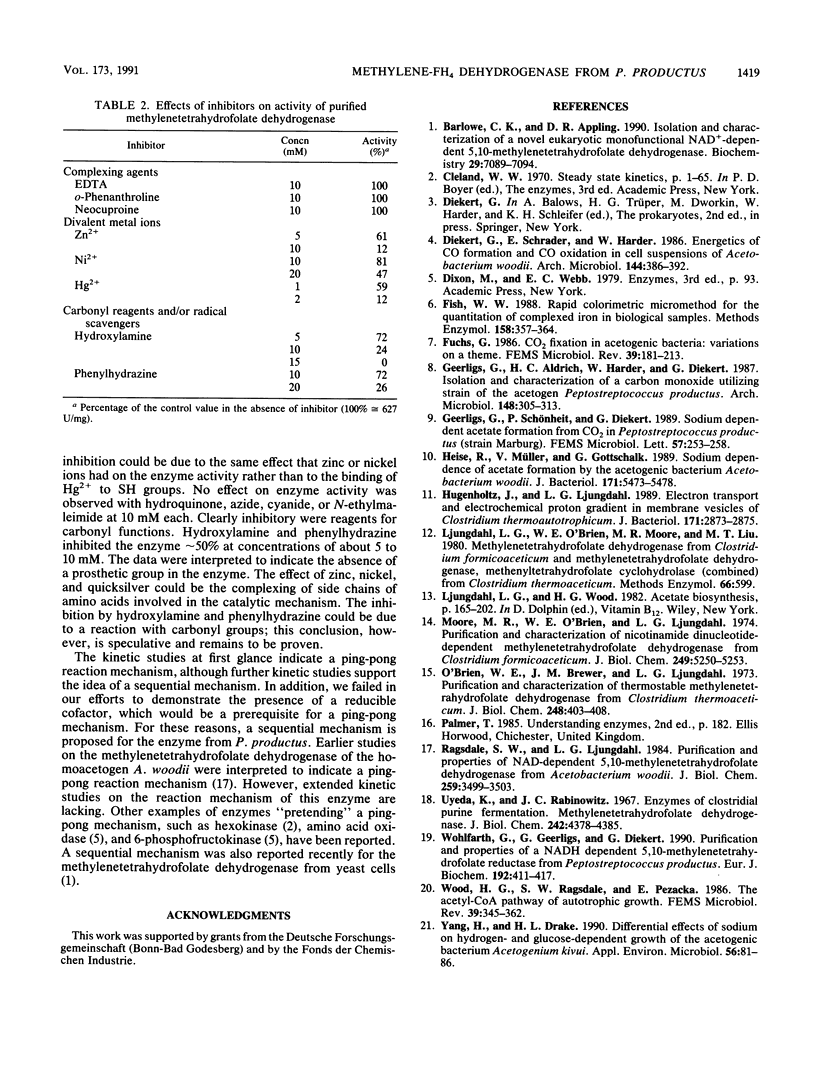
The 5,10-methylenetetrahydrofolate dehydrogenase of heterotrophically grown Peptostreptococcus productus Marburg was purified to apparent homogeneity. The purified enzyme catalyzed the reversible oxidation of methylenetetrahydrofolate with NADP+ as the electron acceptor at a specific activity of 627 U/mg of protein. The Km values for methylenetetrahydrofolate and for NADP+ were 27 and 113 microM, respectively. The enzyme, which lacked 5,10-methenyltetrahydrofolate cyclohydrolase activity, was insensitive to oxygen and was thermolabile at temperatures above 40 degrees C. The apparent molecular mass of the enzyme was estimated by gel filtration to be 66 kDa. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed the presence of a single subunit of 34 kDa, accounting for a dimeric alpha 2 structure of the enzyme. Kinetic studies on the initial reaction velocities with different concentrations of both substrates in the absence and presence of NADPH as the reaction product were interpreted to indicate that the enzyme followed a sequential reaction mechanism. After gentle ultracentrifugation of crude extracts, the enzyme was recovered to greater than 95% in the soluble (supernatant) fraction. Sodium (10 microM to 10 mM) had no effect on enzymatic activity. The data were taken to indicate that the enzyme was similar to the methylenetetrahydrofolate dehydrogenases of other homoacetogenic bacteria and that the enzyme is not involved in energy conservation of P. productus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlowe C. K., Appling D. R. Isolation and characterization of a novel eukaryotic monofunctional NAD(+)-dependent 5,10-methylenetetrahydrofolate dehydrogenase. Biochemistry. 1990 Jul 31;29(30):7089–7094. doi: 10.1021/bi00482a020. [DOI] [PubMed] [Google Scholar]
- Fish W. W. Rapid colorimetric micromethod for the quantitation of complexed iron in biological samples. Methods Enzymol. 1988;158:357–364. doi: 10.1016/0076-6879(88)58067-9. [DOI] [PubMed] [Google Scholar]
- Geerligs G., Schönheit P., Diekert G. Sodium dependent acetate formation from CO2 in Peptostreptococcus products (strain Marburg). FEMS Microbiol Lett. 1989 Feb;57(3):253–257. doi: 10.1016/0378-1097(89)90309-1. [DOI] [PubMed] [Google Scholar]
- Heise R., Müller V., Gottschalk G. Sodium dependence of acetate formation by the acetogenic bacterium Acetobacterium woodii. J Bacteriol. 1989 Oct;171(10):5473–5478. doi: 10.1128/jb.171.10.5473-5478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Ljungdahl L. G. Electron transport and electrochemical proton gradient in membrane vesicles of Clostridium thermoautotrophicum. J Bacteriol. 1989 May;171(5):2873–2875. doi: 10.1128/jb.171.5.2873-2875.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G., O'Brien W. E., Moore M. R., Liu M. T. Methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum and methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate cyclohydrolase (combined) from Clostridium thermoaceticum. Methods Enzymol. 1980;66:599–609. doi: 10.1016/0076-6879(80)66513-6. [DOI] [PubMed] [Google Scholar]
- Moore M. R., O'Brien W. E., Ljungdahl L. G. Purification and characterization of nicotinamide adenine dinucleotide-dependent methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum. J Biol Chem. 1974 Aug 25;249(16):5250–5253. [PubMed] [Google Scholar]
- O'Brien W. E., Brewer J. M., Ljungdahl L. G. Purification and characterization of thermostable 5,10-methylenetetrahydrofolate dehydrogenase from Clostridium thermoaceticum. J Biol Chem. 1973 Jan 25;248(2):403–408. [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G. Purification and properties of NAD-dependent 5,10-methylenetetrahydrofolate dehydrogenase from Acetobacterium woodii. J Biol Chem. 1984 Mar 25;259(6):3499–3503. [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Enzymes of clostridial purine fermentation. Methylenetetrahydrofolate dehydrogenase. J Biol Chem. 1967 Oct 10;242(19):4378–4385. [PubMed] [Google Scholar]
- Wohlfarth G., Geerligs G., Diekert G. Purification and properties of a NADH-dependent 5,10-methylenetetrahydrofolate reductase from Peptostreptococcus productus. Eur J Biochem. 1990 Sep 11;192(2):411–417. doi: 10.1111/j.1432-1033.1990.tb19242.x. [DOI] [PubMed] [Google Scholar]
- Yang H. C., Drake H. L. Differential effects of sodium on hydrogen- and glucose-dependent growth of the acetogenic bacterium Acetogenium kivui. Appl Environ Microbiol. 1990 Jan;56(1):81–86. doi: 10.1128/aem.56.1.81-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


