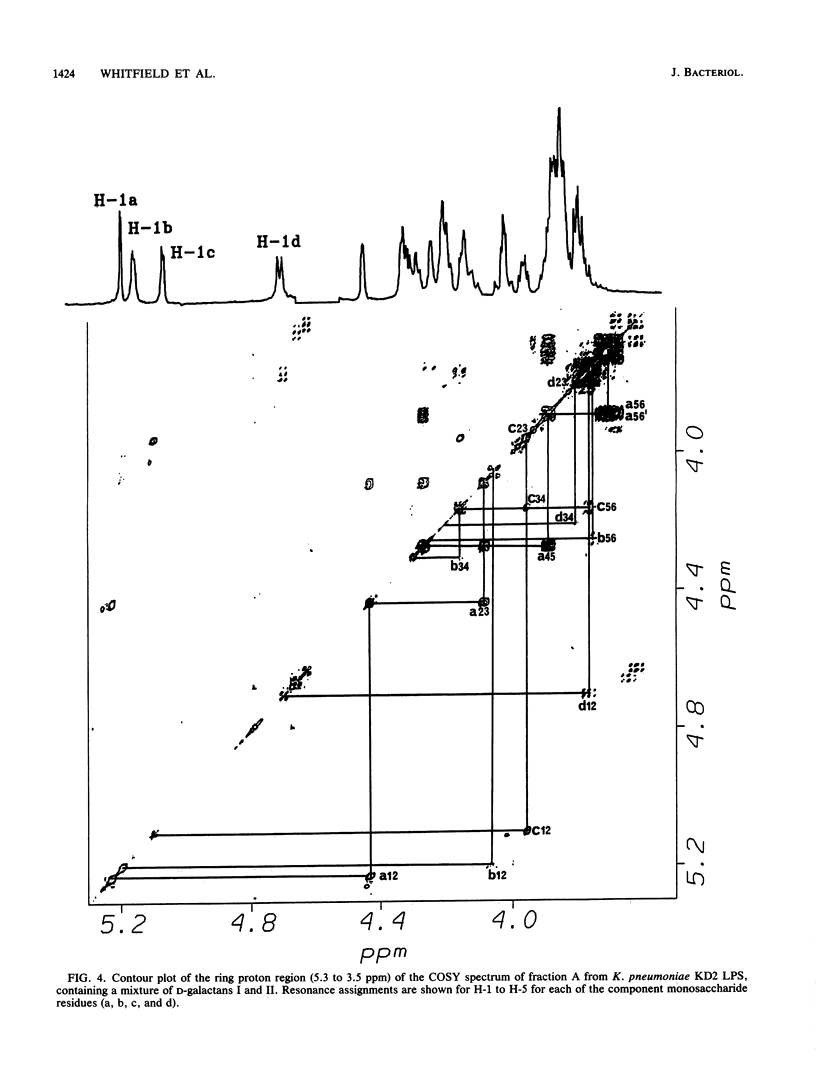
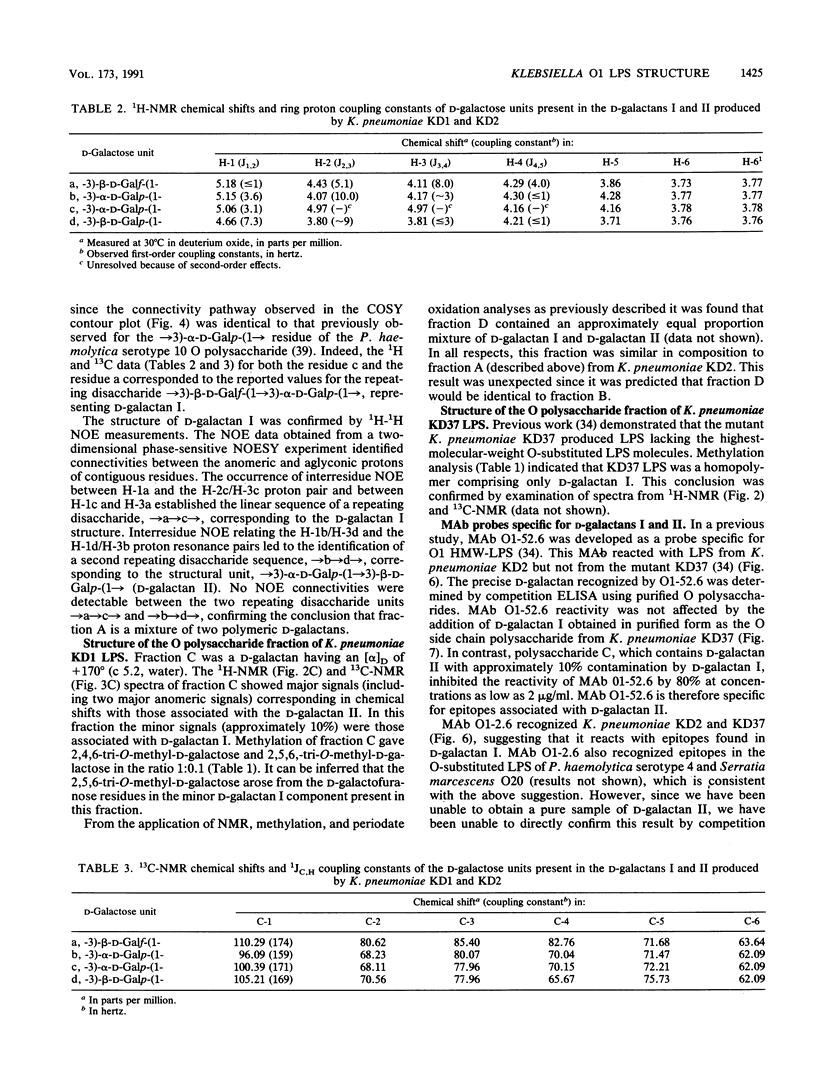
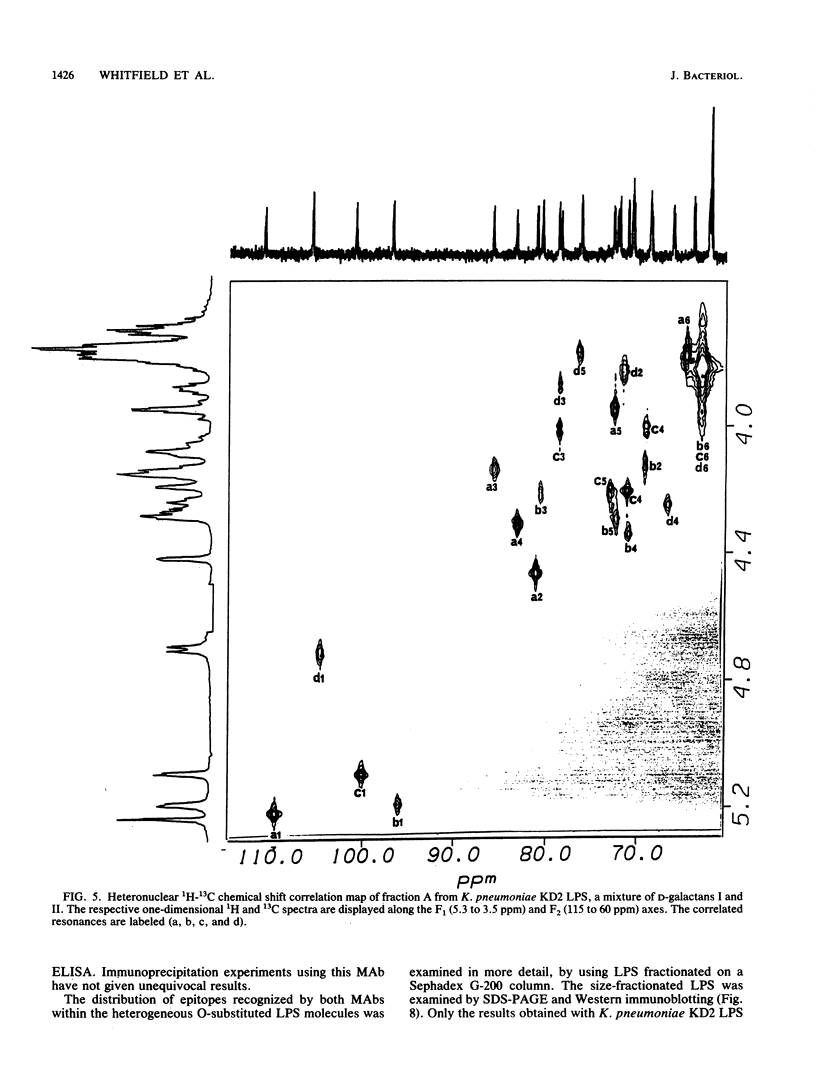
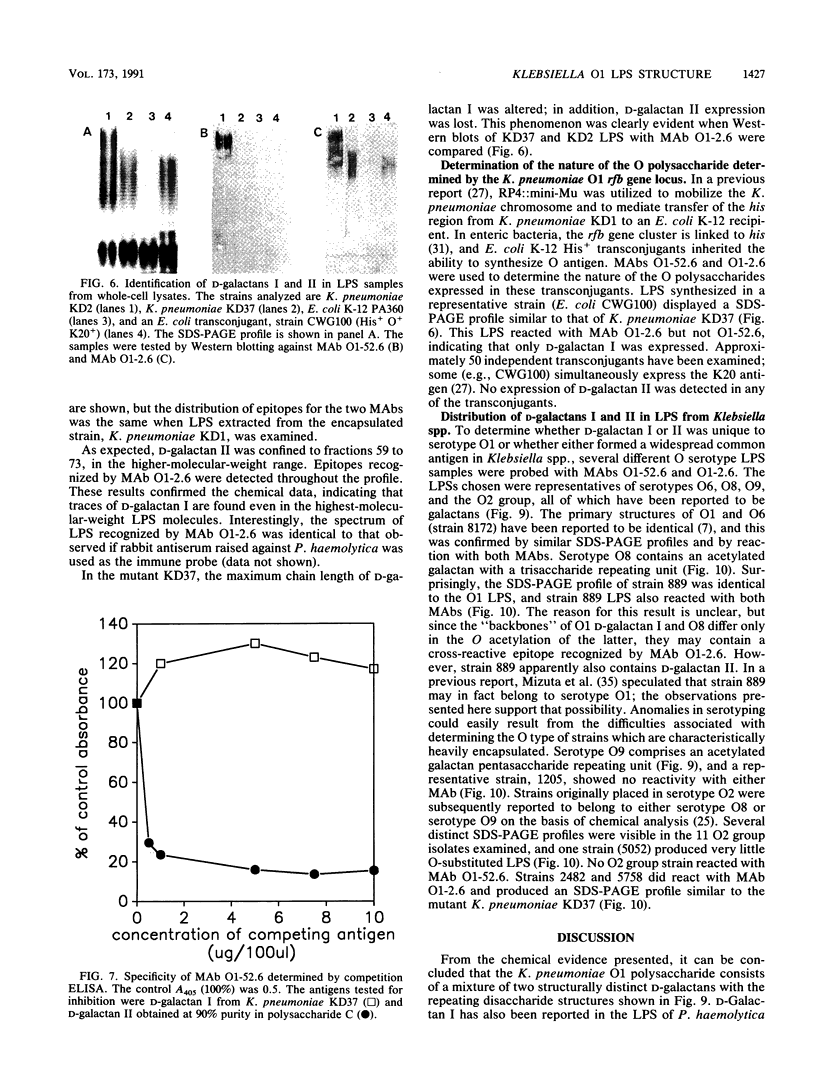
Abstract
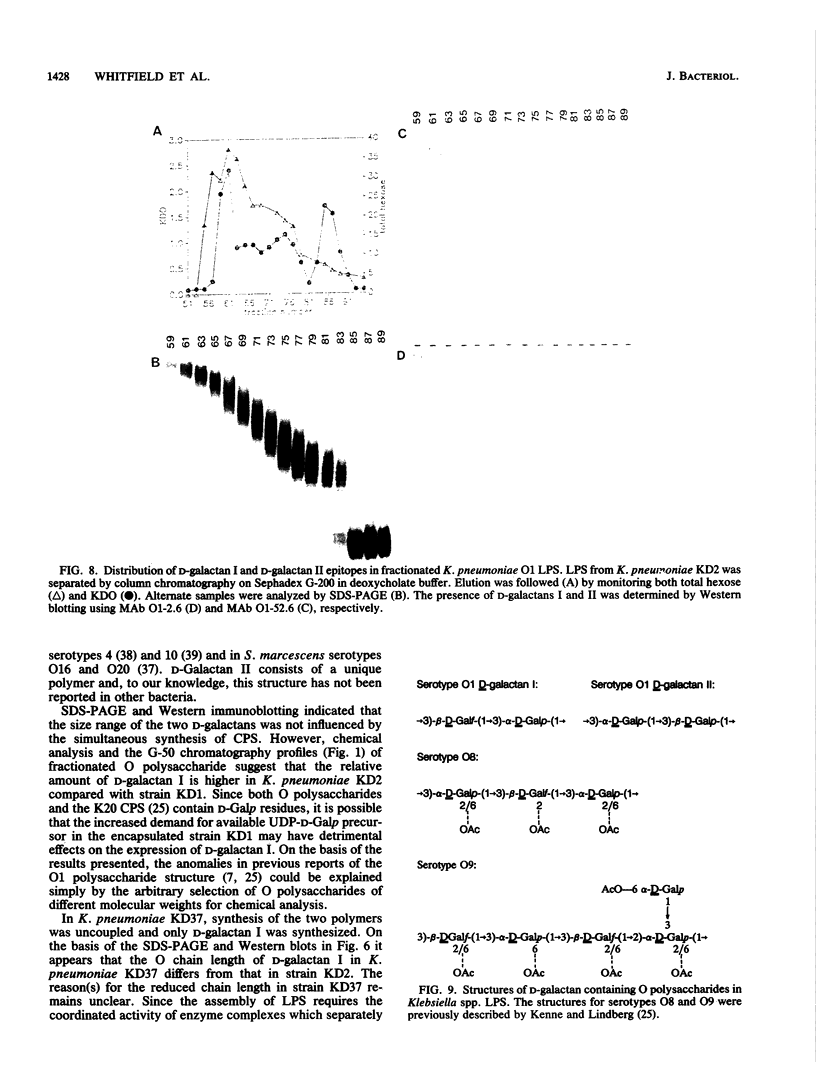
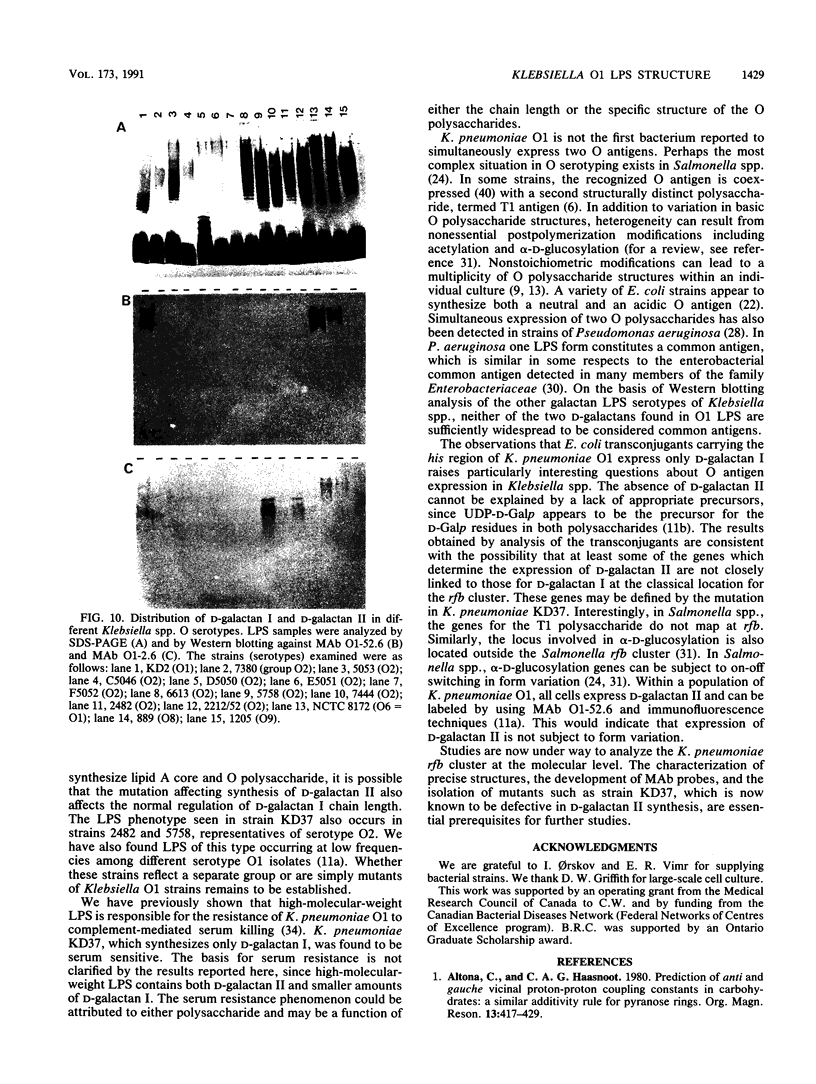
The lipopolysaccharide (LPS) molecule is an important virulence determinant in Klebsiella pneumoniae. Studies on the serotype O1 LPS were initiated to determine the basis for antigenic heterogeneity previously observed in the O1 side chain polysaccharides and to resolve apparent ambiguities in the reported polysaccharide structure. Detailed chemical analysis, involving methylation and 1H- and 13C-nuclear magnetic resonance studies, demonstrated that the O-side chain polysaccharides of serotype O1 LPS contained a mixture of two structurally distinct D-galactan polymers. The repeating unit structures of these two polymers were identified as [----3)-beta-D-Galf-(1----3)-alpha-D-Galp-(1----] (D-galactan I) and [----3)-alpha-D-Galp-(1----3)-beta-D-Galp-(1----] (D-Galactan II). D-Galactan I polysaccharides were heterogeneous in size and were detected throughout the sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) profile of O1 LPS. In contrast, D-galactan II was confined to the higher-molecular-weight region. The structures of the two D-galactans were not influenced by simultaneous synthesis of a capsular K antigen. Apparently, neither of the D-galactans constitutes a common antigen widespread in Klebsiella spp. as determined by immunochemical analysis. Examination of the LPSs in mutants indicated that expression of D-galactan I can occur independently of D-galactan II. Transconjugants of Escherichia coli K-12 strains carrying the his region of K. pneumoniae were constructed by chromosome mobilization with RP4::mini-Mu. In these transconjugants, the O antigen encoded by the his-linked rfb locus was determined to be D-galactan I, suggesting that genes involved in the expression of D-galactan II are not closely linked to the rfb cluster.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berst M., Lüderitz O., Westphal O. Studies on the structure of T1 lipopolysaccharides. Eur J Biochem. 1971 Feb 1;18(3):361–368. doi: 10.1111/j.1432-1033.1971.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Björndal H., Lindberg B., Nimmich W. Structural studies on Klebsiella O groups 1 and 6 lipopolysaccharides. Acta Chem Scand. 1971;25(2):750–750. doi: 10.3891/acta.chem.scand.25-0750. [DOI] [PubMed] [Google Scholar]
- Brisson J. R., Perry M. B. The structures of the two lipopolysaccharide O-chains produced by Salmonella boecker. Biochem Cell Biol. 1988 Oct;66(10):1066–1077. doi: 10.1139/o88-123. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fabio J. L., Brisson J. R., Perry M. B. Structural analysis of the three lipopolysaccharides produced by Salmonella madelia (1,6,14,25). Biochem Cell Biol. 1989 Feb-Mar;67(2-3):78–85. doi: 10.1139/o89-012. [DOI] [PubMed] [Google Scholar]
- Domenico P., Diedrich D. L., Straus D. C. Extracellular polysaccharide production by Klebsiella pneumoniae and its relationship to virulence. Can J Microbiol. 1985 May;31(5):472–478. doi: 10.1139/m85-088. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J., Work E. A colorimetric estimation of lipopolysaccharides. FEBS Lett. 1971 Sep 1;16(4):343–345. doi: 10.1016/0014-5793(71)80386-1. [DOI] [PubMed] [Google Scholar]
- Jann B., Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Kumar A., Ernst R. R., Wüthrich K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem Biophys Res Commun. 1980 Jul 16;95(1):1–6. doi: 10.1016/0006-291x(80)90695-6. [DOI] [PubMed] [Google Scholar]
- Laakso D. H., Homonylo M. K., Wilmot S. J., Whitfield C. Transfer and expression of the genetic determinants for O and K antigen synthesis in Escherichia coli O9:K(A)30 and Klebsiella sp. O1:K20, in Escherichia coli K12. Can J Microbiol. 1988 Aug;34(8):987–992. doi: 10.1139/m88-173. [DOI] [PubMed] [Google Scholar]
- Lam M. Y., McGroarty E. J., Kropinski A. M., MacDonald L. A., Pedersen S. S., Høiby N., Lam J. S. Occurrence of a common lipopolysaccharide antigen in standard and clinical strains of Pseudomonas aeruginosa. J Clin Microbiol. 1989 May;27(5):962–967. doi: 10.1128/jcm.27.5.962-967.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre S., Lucken R., Owen P. Smooth lipopolysaccharide is the major protective antigen for mice in the surface extract from IATS serotype 6 contributing to the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986 Apr;52(1):76–84. doi: 10.1128/iai.52.1.76-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion D., Wüthrich K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem Biophys Res Commun. 1983 Jun 29;113(3):967–974. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- McCallum K. L., Laakso D. H., Whitfield C. Use of a bacteriophage-encoded glycanase enzyme in the generation of lipopolysaccharide O side chain deficient mutants of Escherichia coli O9:K30 and Klebsiella O1:K20: role of O and K antigens in resistance to complement-mediated serum killing. Can J Microbiol. 1989 Nov;35(11):994–999. doi: 10.1139/m89-166. [DOI] [PubMed] [Google Scholar]
- McCallum K. L., Schoenhals G., Laakso D., Clarke B., Whitfield C. A high-molecular-weight fraction of smooth lipopolysaccharide in Klebsiella serotype O1:K20 contains a unique O-antigen epitope and determines resistance to nonspecific serum killing. Infect Immun. 1989 Dec;57(12):3816–3822. doi: 10.1128/iai.57.12.3816-3822.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta K., Ohta M., Mori M., Hasegawa T., Nakashima I., Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 1983 Apr;40(1):56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomerie J. Z. Epidemiology of Klebsiella and hospital-associated infections. Rev Infect Dis. 1979 Sep-Oct;1(5):736–753. doi: 10.1093/clinids/1.5.736. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Mayer H. Enterobacterial common antigen. Bacteriol Rev. 1976 Sep;40(3):591–632. doi: 10.1128/br.40.3.591-632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley D., Wilkinson S. G. Structures of neutral glycans isolated from the lipopolysaccharides of reference strains for Serratia marcescens serogroups O16 and O20. Carbohydr Res. 1989 Oct 31;193:241–248. doi: 10.1016/0008-6215(89)85122-5. [DOI] [PubMed] [Google Scholar]
- Richards J. C., Leitch R. A. Elucidation of the structure of the Pasteurella haemolytica serotype T10 lipopolysaccharide O-antigen by n.m.r. spectroscopy. Carbohydr Res. 1989 Mar 15;186(2):275–286. doi: 10.1016/0008-6215(89)84041-8. [DOI] [PubMed] [Google Scholar]
- Sarvas M., Mäkelä P. H. The production, by recombination, of Salmonella forms with both T-1 and O specifities. Acta Pathol Microbiol Scand. 1965;65(4):654–656. doi: 10.1111/apm.1965.65.4.654. [DOI] [PubMed] [Google Scholar]
- Straus D. C., Atkisson D. L., Garner C. W. Importance of a lipopolysaccharide-containing extracellular toxic complex in infections produced by Klebsiella pneumoniae. Infect Immun. 1985 Dec;50(3):787–795. doi: 10.1128/iai.50.3.787-795.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. C. Production of an extracellular toxic complex by various strains of Klebsiella pneumoniae. Infect Immun. 1987 Jan;55(1):44–48. doi: 10.1128/iai.55.1.44-48.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás J. M., Benedí V. J., Ciurana B., Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986 Oct;54(1):85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Van Gijsegem F., Toussaint A. Chromosome transfer and R-prime formation by an RP4::mini-Mu derivative in Escherichia coli, Salmonella typhimurium, Klebsiella pneumoniae, and Proteus mirabilis. Plasmid. 1982 Jan;7(1):30–44. doi: 10.1016/0147-619x(82)90024-5. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]