Abstract
The bacteriophage P1 hot gene product is a homolog of the θ subunit of E. coli DNA polymerase III. Previous studies with hot cloned on a plasmid have shown that Hot protein can substitute for θ, as evidenced by its stabilizing effect on certain dnaQ mutator mutants carrying an unstable pol III proofreading subunit (ε subunit). These results are consistent with Hot, like θ, being a replication protein involved in stabilizing the intrinsically unstable ε proofreading function. However, the function of hot for the viral life cycle is less clear. In the present study, we show that the hot gene is not essential. Based on its promoter structure, hot has been previously classified as a “late” phage gene, a property that is not easily reconciled with a presumed replication function. Here, we clarify this issue by demonstrating that P1 hot is actively expressed both during the lysogenic state and in the early stages of a lytic induction, in addition to its expression in the late stage of phage development. The results indicate that P1 hot has a complex expression pattern, compatible with a model in which Hot may affect the host replication machinery to benefit overall phage replication.
Keywords: DNA polymerase III, θ subunit, Bacteriophage P1, P1 Hot protein, Mutator effects
1. Introduction
The bacteriophage P1 hot gene encodes a homolog of the θ subunit of E. coli DNA polymerase III holoenzyme, which is responsible for replicating the bacterial chromosome (for reviews, see [1–3]). Specifically, θ is a component of the pol III core, which consists of the α subunit (DNA polymerase), the ε subunit (3′ exonuclease that serves as proofreader for Pol III insertion errors), and θ, which are tightly bound together in the linear order α–ε–θ. Our laboratory has focused on the possible function of the θ subunit within the pol III core. The subunit is not essential, as strains bearing a deletion of holE, the gene that encodes θ, are normally viable [4]. However, ΔholE strains were shown to possess a modest mutator phenotype, indicating that θ has a role in replication fidelity, most likely through its effect on the ε proofreading subunit [5]. We further proposed a stabilizing role for θ on ε subunit based on observations that certain dnaQ mutator mutants whose mutator phenotype resulted from a temperature-sensitive or otherwise unstable ε proofreading subunit were greatly destabilized in the absence of θ. For example, at low temperature where the mutability of the temperature-sensitive dnaQ49 strain is normally minimal, its mutator activity is increased some 1,000-fold by the absence of θ [5].
The discovery that the genome of bacteriophage P1 encodes a homolog of θ, named hot (homolog of theta) [6], has raised some interesting issues. Phage P1 relies on the host replication machinery and does not encode any other component of the Pol III holoenzyme, although it encodes at least two replication-associated activities, a DNA helicase (ban) and a single-stranded binding protein (ssb) [6–8]. This raises the question of the possible function of hot in the phage life cycle. By implication, an answer to this question is likely to bear on the precise role of θ in E. coli. Genetic studies have shown that hot, when cloned and expressed from the holE promoter on either a low-copy plasmid or the E. coli chromosome, is able to complement a ΔholE defect, reducing, for example, the ΔholE dnaQ49 mutator effect by some 1000-fold [9]. NMR structural studies on both θ and Hot revealed the two proteins to have near identical structures [10–12], further consistent with Hot being a fully functional homolog of θ. Alignment of the two proteins shows them to be approximately 48–53% identical (60–66% similar), which increases to 70 and 80%, respectively, when considering the structured core of the molecules [13].
Despite the finding that Hot can substitute for θ, no information exists about whether hot is expressed from the phage genome at any point in the phage life cycle. Of interest in this respect is the previous description of hot as a “late” viral gene [6,14,15]. This classification as a late gene is based on its unusual promoter structure (containing a −10 and −22 consensus sequence rather than the normal −10 and −35 sequence), which has been associated with expression of late phage genes [14,15]. This implies that Hot might not be expressed until the phage packaging stage when DNA replication has largely ceased, a somewhat unusual expression profile for a presumed replication protein. Presently, we report on several experiments addressing these issues. We first create a phage containing a deletion of the hot gene and show that this phage is normally viable. We then demonstrate that hot is expressed in the lysogenic stage as well as in both early and late stages of a lytic cycle.
2. Materials and methods
2.1. Strains and media
E. coli strains NR17116 (dnaQ49 zae-502::Tn10), NR16328 (dnaQ930 zae-502::Tn10), NR16320 (ΔholE dnaQ923 zae-502::Tn10), and NR16329 (ΔholE dnaQ930 zae-502::Tn10) are derivatives of strain MG1655 [16] and have been described [13]. KA796 (ara, thi, Δprolac) has also been described [17]. P1 bacteriophage c1–100 Tn9 used throughout this study has been described [6,9]. Strains BW25113 and BT430 [pCP20] and plasmids pKD4 and pKD46, all used for deletion mutagenesis of P1 by the method of Datsenko and Wanner [18], were obtained from the E. coli Genetic Stock Center (Yale University).
LB medium was used as described [17]. LB plates contained 1.5% agar (Difco). Liquid LB cultures used for phage experiments included 5 mM CaCl2. P1 phage titers were determined using LB plates (bottom agar) supplemented with CaCl2 (2 mM), thymidine (10 μg/ml), and glucose (0.1%). Top agar was 0.3% agar (Difco) in water. Antibiotics, when indicated, were used at 25 μg/ml (kanamycin), 100 μg/ml (ampicillin), or 15 μg/ml (chloramphenicol). LBRif plates used to determine the frequency of rifampicin-resistant E. coli mutants contained 100 μg/ml rifampicin. Antibiotics were obtained from Sigma–Aldrich.
2.2. Construction of P1 derivatives containing Δhot::kan, ΔhumD::kan, or Tn9Δcat::kan mutations
To create deletion/insertion mutations in the P1 genome we used the PCR-mediated method of Datsenko and Wanner [18]. The primers used are described in Table 1: HDKU and HDKL were used to create P1Δhot, HumKU and HumKL were used to create P1ΔhumD, and Cam Tn9 U and Cam Tn9 L were used to inactivate the cat gene of P1-contained transposon Tn9. The primers were used for PCR amplification of plasmid pKD4 [18] leading to amplification of the kanamycin (kan) resistance cassette of pKD4 flanked by FLP recognition sequences [18] and P1 genomic sequences immediately upstream and downstream of the genes to be deleted. Strain BW25113 [18] carrying plasmid pKD46 (AmpR) was infected by bacteriophage P1 c1–100 Tn9 and CamR AmpR lysogens were selected at 30 °C. Competent cells of these lysogens were then used for electroporation with the PCR fragments described above. Selection was for CamR and KanR transformants (or only KanR in case of inactivation of the Tn9 cat gene) at 30 °C. The resulting transformants were tested for the presence of P1 by phage induction at 42 °C and for loss of the target gene by PCR using primers Hot Upper and Hot Lower for hot or HumD Upper and HumD Lower for humD (Table 1). The phage obtained by the thermal induction were then used to create the corresponding lysogens of strain MG1655.
Table 1.
Oligonucleotide primers used in this study
| HDKUa | 5′ gCTggTTAAATAATAgTCgTTACTCAATTATTCTggATgggATTTgATAggCTggAgCTgCTTC 3′ |
| HDKLa | 5′ CAAAACggTTAACTACCgCTATTTCTTTACggCATCATCTTTCTgATATggATCCgTCgACCTgC 3′ |
| HumKUa | 5′ gCTTCCCTTCTCCTgCggCggATTATgTTgAAAgCCgAATTTCTCTTgAggCTggAgCTgCTTC 3′ |
| HumKLa | 5′ gCACCAgCACTTTgCAgCTTAAATgACCggACAATCATCAAACTCTggATCCgTCgACCTgC 3′ |
| Cam Tn9 Ua | 5′ CCgTTgATATATCCCAATggCATCgTAAAgAACATTTTgAggCTggAgCTgCTTC 3′ |
| Cam Tn9 La | 5′ ATTCATCAggCgggCAAgAATgTgAATAAAggCCggATCCgTCgACCTgC 3′ |
| Hole Upb | 5′ gCTgAAgAATCTggCTAAAC 3′ |
| Hole lowb,c | 5′ TTAAgTTTgggCTCgTAAg 3′ |
| HumD upperb | 5′ AAgCCgAATTTCTCTTgATC 3′ |
| HumD lowerb,c | 5′ gACAATCATCAAACTCTCCAC 3′ |
| Hot upperb | 5′ ggATTTgATATgTACgATTgg 3′ |
| Hot lowerb,c | 5′ ggCAACTggAggCTTAAC 3′ |
| Slowd,e | 5′ gTCTCCAgATCCTCCTTgC 3′ |
| Supd | 5′ ggTTTAATCACCggCTTAC 3′ |
Primers used to create deletions of the P1 genes hot (HDKU and HDKL), humD (HumKU and HumKL), and Tn9kan (Cam Tn9 U and Cam Tn9 L) (see text).
Gene-specific primers for genotyping and QPCR of indicated genes.
Gene-specific primers used to prepare cDNA.
Primers used for QPCR of gene 16.
Primer used for gene 16 cDNA preparation.
2.3. FLP-mediated excision of the kan resistance cassette
To eliminate the kan insert from the P1 Δhot::kan and ΔhumD::kan derivatives, MG1655 lysogens containing these phages were induced by temperature shift, and the resulting lysates were used to infect strain BT340 containing plasmid pCP20 [18]. Since pCP20 confers chloramphenicol resistance, no direct selection for lysogens was available in this strain. Therefore, low density cultures of BT340[pCP20] were infected with the lysates and grown at 30 °C overnight in LB + chloramphenicol. The next day, 10 μl of the cultures were transferred into 1 ml of fresh medium and the cultures were grown at 30 °C until OD600 = 0.2. The cultures were then induced by transfer to 43 °C and grown with vigorous shaking until lysis occurred (35–40 min). Chloroform was added to inactivate any surviving bacteria and the resulting lysates were used to infect strain MG1655 to obtain new lysogens (LB + chloramphenicol, 30 °C). The new lysogens were tested for kanamycin resistance, and kanamycin-sensitive colonies were examined by PCR to confirm loss of the kanamycin cassette.
2.4. Analysis of phage titers produced by lysogens
Cultures of phage lysogens were grown overnight at 30 °C in LB + chloramphenicol. The next morning, the cultures were diluted (1:100) in fresh medium (LB + CaCl2). The cultures were grown for 3–4 h at 30 °C until the OD600 reached 0.2 and were then transferred to a 45 °C water bath. After 10 min the temperature was reduced to 42 °C and the cultures were incubated for another 30–35 min with vigorous shaking until lysis was visible. A few drops of chloroform were added to inactivate remaining bacteria. The phage titer was then determined by detection of the number of PFU in soft agar layers using MG1655 as indicator host. Plates were incubated at 40 °C. The visible size of the plaques was maximized by using 0.3% soft agar in the top layer and by optimizing the amount of indicator host. For each phage mutant 10–20 independent cultures were analyzed.
2.5. Bacterial mutant frequencies
To measure mutant frequencies, the frequency of Rifr mutants in overnight cultures was determined. Ten to fifteen cultures for each strain started from individual colonies were grown overnight in 1 ml LB broth + chloramphenicol at 30 °C. For each culture, a 50-μl aliquot of a 106 dilution was spread on an LB plate to determine the total number of viable cells, and 50 μl of the undiluted or appropriately diluted culture was spread on LBRif plates to determine the number of rifampicin-resistant mutants. The mutant frequency for each culture was calculated by dividing the total number of mutants by the total number of viable cells. The data were analyzed using the statistical analysis software Prism (GraphPad).
2.6. Analysis of gene expression by QPCR
Expression analysis of the P1 hot gene and the known late gene 16 as well as the host holE gene was conducted by measurement of the corresponding mRNA levels using Quantitative PCR (QPCR). Cultures of P1 c1–100 Tn9 lysogens of strains MG1655 or KA796 were grown in 1 ml of LB + chloramphenicol at 30 °C overnight. The cultures were diluted 1:100 to 1:500 in 50 ml fresh LB + 5 mM CaCl2 and grown at 30 °C with vigorous shaking until OD600 reached 0.4–0.6. At this point, 50 ml of fresh preheated (45 °C) medium were added to each flask and the cultures were placed on a shaker at 42 °C. This thermal induction of the phage resulted in visible lysis of the cultures after 35–50 min depending on the precise density of the cultures. Every 5–10 min, 2 ml samples of the culture were collected and mixed immediately with 4 ml of RNAprotect Bacteria reagent (Qiagen). A zero-time sample was also taken directly before the temperature shift. The samples were centrifuged (10 min at 5000 × g) and the obtained pellets were used for purification of total RNA using a RNeasy Mini Kit (Qiagen) as recommended by the manufacturer. The RNA was treated with RNAse-free DNAse Set (Qiagen) for 1 h at room temperature. DNAse was inactivated by heating for 15 min at 70 °C. The RNA amounts were quantitated using the Quant-iT RiboGreen RNA Assay Kit (Invitrogen). To generate the standard curve, multiple dilutions of the ribosomal RNA were used starting at 2.5 ng/μl. Dilutions were prepared in 96-well plates and analyzed on a Mx3000P QPCR machine (Stratagene), as recommended by the manufacturer. Based on these results, all RNA samples were diluted to the same final concentration (30–70 ng/μl in different experiments). Five to ten microliters of the RNA samples were then converted into cDNA by reverse transcription using SuperScript II reverse transcriptase (Invitrogen) and either random hexamer primers or gene-specific primers (Table 1). The reaction was run as recommended by Invitrogen for 1 h; the temperatures were room temperature for the random hexamers and 42 °C for the gene specific primers. For each sample, control reactions without reverse transcriptase were also performed to check for any residual DNA contamination. Two to ten microliters of the cDNA preparation was taken into a QPCR reaction run on a Mx3000P QPCR instrument (Stratagene) using the SYBR Green PCR Master Mix (Applied Biosystems) and the gene-specific primers listed in Table 1. Equal sample volumes were also taken from the “no reverse transcriptase” controls to verify the lack of residual phage or chromosomal DNA in the RNA samples. The PCR temperature profile used was: 10 min at 95 °C, followed by 40 cycles of 94 °C (30 s), 61 °C (70 s), and 72 °C (40 s); finally, a dissociation curve cycle. Primer concentrations were 200 μM each. For each primer pair, standard curves were generated using serial four-fold dilutions of purified DNA from each indicated P1 lysogen. The gene specific primers were Hot Upper and Hot Lower primers for detection of hot cDNA, Slow and Sup used for detection of gene 16 cDNA, and holE Up and holE Low for detection of host holE cDNA. For each reaction, the correctness of the PCR product was checked using the dissociation curve cycle option on the QPCR instrument. Statistical analysis was done using “Prism” software (GraphPad).
3. Results
3.1. The P1 hot gene is not essential
As a first step in analyzing the role of the hot gene in the life of phage P1, we investigated whether it was possible to create a phage carrying a deletion of the hot gene. For this and other experiments in this report we used P1 c1–100 Tn9. This phage carries a temperature-sensitive (c1) repressor, facilitating experimental control of the lysogeny/lytic development switch. It also carries transposon Tn9 specifying chloramphenicol resistance [6]. This combination allows ready creation and maintenance of chloramphenicol-resistant lysogens at 30°. We used the method of Datsenko and Wanner [18] to convert the prophage into a Δhot::kan prophage in which the hot gene is replaced by a kanamycin-resistance cassette [18] (see Section 2). As controls, we also created ΔhumD::kan and Tn9Δcat::kan phages. The humD gene is adjacent to hot and encodes a homolog of the E. coli UmuD protein [6,19]. Deletion of the cat gene of Tn9 creates a chloramphenicol-sensitive, kanamycin-resistant phage derivative. The deletion/insertion products were confirmed by PCR analysis. Each of the three kanamycin-resistant lysogens produced phage upon thermal induction, and phage derived from these inductions could be used to generate new lysogens at 30°.
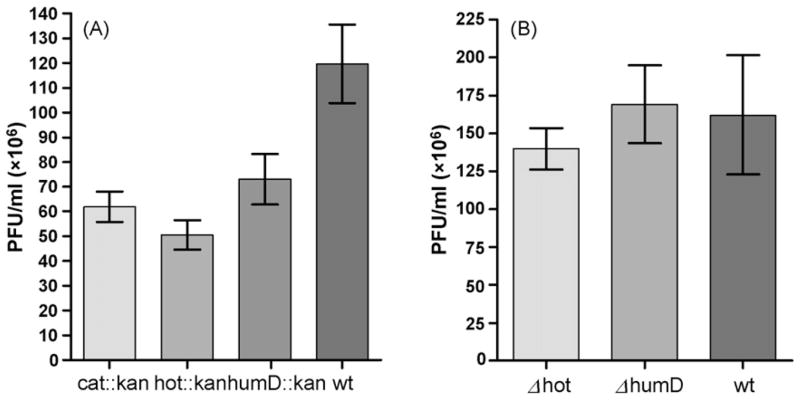
These results indicate that hot is not essential for either prophage establishment/maintenance or for lytic development. On the other hand, we noted that phage titers obtained after induction of all three lysogens were reproducibly two- to three-fold lower than obtained from the parental prophage, and were accompanied by smaller plaque sizes and lower yields of lysogens (Fig. 1A). These effects were likely related to the insertion of the kan gene into the P1 genome, because when the kan cassette was eliminated by FLP-mediated excision (see Section 2), titers and other phage properties returned to normal (Fig. 1B). To check for a possible requirement for hot in the absence of the host θ subunit, we also investigated the various properties of the Δhot phage in the ΔholE strain NR13104 [9]. No significant differences between the Δhot and hot+ phages were found (data not shown).
Fig. 1.
Phage yields (PFU) after thermal induction of P1 lysogens. (A) Phages carrying a kanamycin-resistance cassette (kan) in three different P1 loci (Tn9 (cat), hot, or humD) as detailed in the text. ‘wt’ represents the parental P1 c1–100 Tn9 lysogen. Induction and titering were performed as described in Section 2. (B) As in (A) but after removal of the kan insert from hot or humD, indicated as Δhot or ΔhumD. The results represent the mean value (±S.E.M.) for 20 independent cultures for each indicated strain as calculated by “Prism” software.
3.2. Expression of hot in P1 lysogens
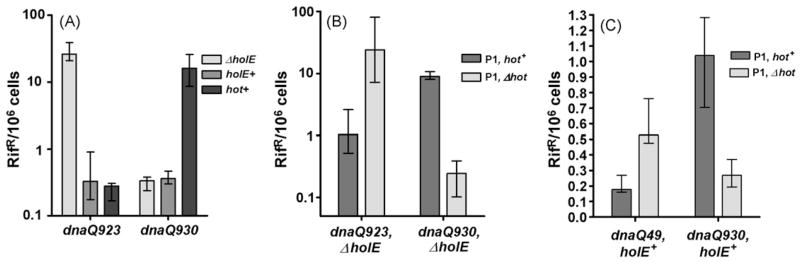
To investigate the expression of hot from the phage genome, we first checked its expression from the prophage during lysogeny. We made use of our previous observations that Hot, when cloned and expressed from a low-copy plasmid, is capable of strongly reducing the mutability of several unstable dnaQ mutator mutants, such as dnaQ49 or dnaQ923 [9]. Interestingly, at the same time it is capable of strongly increasing the mutability of another dnaQ mutant, dnaQ930 [13]. The precise reason for this mutator effect of Hot is unknown, but likely reflects a slightly different interaction of θ and Hot with ε that exacerbates the dnaQ930 defect (H98Y) [13,20]. This dual phenomenology permits a convenient test for expression of hot from the P1 prophage by checking whether these mutator/antimutator effects can be reproduced by a resident prophage. We created lysogens of P1 and P1 Δhot of strains NR16320 (ΔholE dnaQ923) and NR16329 (ΔholE dnaQ930) [13], selecting for chloramphenicol-resistant derivatives at 30°, and measured the frequency of rifampicin-resistant mutants. In Fig. 2A we reproduce the effects of hot when expressed from the bacterial chromosome in place of holE as reported before [13]. For the dnaQ923 strain lacking θ (ΔholE), the mutant frequency is high but is significantly reduced in the presence of either θ (holE+) or P1 Hot (hot+). In contrast, for dnaQ930 the mutant frequency is modest without or with θ, but increases by 10-fold by the presence of Hot. Fig. 2B shows that these effects of Hot are also observed when the hot gene is present on a resident P1 prophage. Comparing the hot+ and Δhot phages, the presence of hot clearly decreases the mutator effect of dnaQ923 and increases the mutator effect of dnaQ930. Thus, the hot gene is clearly expressed from the phage genome during the lysogenic state.
Fig. 2.
Mutator or antimutator effect of Hot protein produced by P1 lysogens, as monitored by the frequency of rifampicin-resistant (RifR) mutants. (A) Control experiment for dnaQ923 or dnaQ930 strains containing ΔholE, holE+, or P1 hot on the E. coli chromosome. The strains were NR16320 (dnaQ923, ΔholE), NR16319 (dnaQ923), NR16321 (dnaQ923, ΔholE::hot), NR16329 (dnaQ930, ΔholE), NR16328 (dnaQ930), and NR16330 (dnaQ930, ΔholE::hot) as described previously [13]. For comparison, typical RifR mutant frequencies for non-mutator (dnaQ+) strains are (0.02–0.05) × 106. (B) Effect of Hot protein on dnaQ923 and dnaQ930 strains when the hot gene is expressed from the P1 prophage. Strains NR16320 (dnaQ923 ΔholE) and NR16329 (dnaQ930 ΔholE) [13] were infected by bacteriophage P1 c1–100 Tn9 and its Δhot derivative to create lysogens. Fifteen independent lysogens for each strain were used to measure the frequency of rifampicin-resistant mutants. Cultures grown overnight at 30 °C in LB + chloramphenicol. The graph shows median values and the interquartile ranges for the frequency of rifampicin-resistant mutants as calculated by the statistical analysis software Prism (GraphPad). (C) As in (B) above, but here the bacterial hosts are holE+ strains NR17116 (dnaQ49) and NR16328 (dnaQ930) [13].
Previously we also showed that hot, when expressed from a plasmid, was capable of competing with θ for incorporation into Pol III [9,13]. Therefore, we also tested if expression from the P1 prophage is sufficient to compete with θ. This was tested with the dnaQ49 mutator, which, like dnaQ923, is strongly stabilized by both θ and Hot [9]. In particular, the antimutagenic effect were of Hot for dnaQ49 is several-fold stronger than that of θ [9,13], making it a useful allele for studying competition of Hot with θ. We infected the holE+ strains NR17116 (dnaQ49) and NR16328 (dnaQ930) [13] with the two types of P1 and assayed RifR mutant frequencies. The results shown in Fig. 2C indicate that, also under these conditions, the hot+ prophage was capable of reducing the mutant frequency of the dnaQ49 strain while increasing the mutant frequency of the dnaQ930 strain. The results of these experiments clearly indicate that Hot protein is expressed by P1 lysogens, and at levels sufficiently high to compete with the endogenous θ protein for incorporation in the Pol III core and HE.
3.3. Hot expression during the P1 lytic cycle
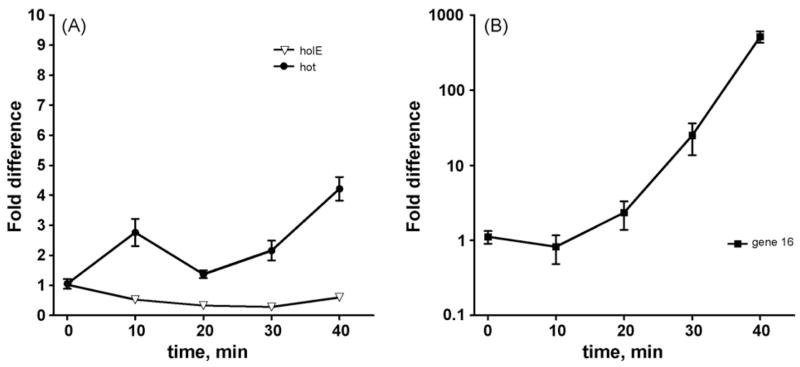
In the experiments described above we also checked for the presence of hot mRNA in the lysogens using RT PCR and hot-specific primers. These experiments confirmed the presence of this mRNA (data not shown). We then investigated the expression of hot mRNA during a lytic cycle initiated by a temperature shift up using Quantitative PCR (QPCR) (see Section 2). Lysis of the cultures was generally observed between 35 and 50 min after the shift. At various times after the shift, but before lysis, samples were withdrawn and the amount of hot mRNA was determined by conversion to cDNA and quantitation of the cDNA by QPCR. In parallel, the RNA samples were used to determine expression of a known P1 late gene, gene 16, encoding the P1 base plate or tail tube [6], as well as expression of the chromosomal holE gene. In the lysogen (zero time), hot mRNA was readily detected, whereas gene 16 mRNA was either undetectable or present at very low levels. In Fig. 2 we show the results of a representative experiment. The figure presents the calculated changes in specific mRNA levels upon phage induction relative to the starting point. hot mRNA was induced significantly at the 10 min time point. Other experiments (not shown here) indicated that hot mRNA is increased by 2.5- to 4-fold as early as 5 min after thermal induction. At the 20 min time point, hot is reproducibly reduced close to the levels detected in the lysogen. At the 30-min point it is increased again, and at 40 min time point it is four- to eight-fold elevated compared to the lysogenic stage. This reproducible pattern is very different from that of gene 16, which behaves like a typical late gene. Induction of gene 16 starts around 20 min, and it is induced 600- to 1000-fold at the 40 min time point. The amount of holE mRNA was largest at the 0 time point and declined thereafter by two- to four-fold. This decline may result most simply from dilution of host mRNA within the RNA samples due to a strong induction of phage mRNA. This experiment was repeated a total of seven times in two different E. coli backgrounds, MG1655 and KA796 [9,13]. While the absolute numbers varied between the individual experiments, presumably due to small variations in the initial culture densities, the expression patterns for each of the genes as shown in Fig. 3 were highly reproducible.
Fig. 3.
Expression of hot during a P1 lytic cycle. QPCR methods were used to assay expression of the P1 hot gene upon thermal induction of P1 c1–100 Tn9 in strain MG1655 along with that of the known late gene 16 and the chromosomal holE gene. RNA and cDNA samples were prepared as described in Section 2. RNA was quantified using RiboGreen reagent (Invitrogen) and, for each sample, an identical amount of RNA was carried into the cDNA preparation step. Standard QCPR curves were obtained by serial dilutions of purified DNA samples, establishing that, under the conditions used, one cycle of PCR reflected a doubling of the DNA amounts. The Ct (threshold cycle) values obtained from the real-time PCR analysis were converted into the fold increases or decreases displayed on the Y-axis as follows. At each time point, the Ct value was subtracted by the starting value Ct0, yielding (Ct − Ct0) = n, representing the number of cycles by which the PCR product reached the threshold “earlier”. n was then converted to a corresponding cDNA increase by 1/2n, which is represented on the Y-axis. A melting curve experiment was also included as part of each run to verify the correctness of the amplified DNA product (Stratagene). The resulting numbers were analyzed by “Prism” software (GraphPad), and each point on the graph represents the mean value (±S.E.M.). In the indicated (representative) experiment, each data point represents the average of six samples (two independent cultures analyzed in triplicate). The average Ct0 values were 23.2 for holE, 27.3 for hot, and 33.7 for gene 16. Note that due to the large effects for gene 16 expression (panel B), the data for this gene are shown on a logarithmic scale.
4. Discussion
The current results provide new information on the possible role of the bacteriophage P1 hot gene. Previously, this gene was indicated to be a late phage gene [14,15], a possibility not readily reconciled with a replication function for the Hot protein. Our present results clearly indicate that hot is not a typical late gene like, as exemplified, for example, by gene 16 in this study. On the other hand, hot expression does not resemble that of the typical early genes, which are normally induced rapidly early in the lytic phase and suppressed at the late lytic stage [21]. Furthermore, we also demonstrate that hot is active during the lysogenic state. Thus, hot likely has a more complicated mode of regulation permitting expression during each of three phage stages: prophage and early and late lytic phases (at least when the lytic cycle is induced by a temperature shift up), and Hot protein may play a function in all three stages.
The previous studies classifying hot as a late gene were based on its promoter structure, containing −22 and −10 elements, rather than −35 and −10 elements, which serve as recognition elements for the late-gene specific P1 transcription factor gp10 [6,14,15]. The hot gene is unusual among this group of genes in that the spacing between the −10 and −22 regions is nine instead of the canonical four [6]. Thus, this lack of consensus may contribute to the broad expression pattern observed here. It may well be that hot is subject to additional transcription from other promoters, and a further analysis of the precise nature of hot transcripts will be required to address this issue.
Our experiments have shown that in P1-infected cells Hot protein is incorporated in the Pol III core and HE, competing effectively with the host θ subunit. Thus, one might speculate that phage replication, during plasmid maintenance and/or during lytic development, benefits from this substitution. As one possibility, we have suggested [9,13] that the presence of Hot leads to increased availability of the intrinsically unstable ε subunit [22] in the form of ε-Hot complex, leading to increased availability of Pol III core and Pol III HE. This might benefit phage replication, as only a very limited number of HE molecules are thought to be present in E. coli [23,24]. In the present experiments, we did not observe a significant difference in phage yield upon the thermal induction between hot+ and hot− phages (Fig. 1). However, the question of any advantage of Hot may need to be addressed on a longer-term (or evolutionary) scale and should include consideration of all stages of the phage life cycle.
Hot protein, even when the hot gene is present on the single copy P1 prophage, is capable of promoting a strong mutator effect on a host dnaQ930 mutant (Fig. 2B). This effect is similar to that of the cloned hot gene present on a low-copy plasmid [13]. In the latter case, Hot protein was also capable of causing a modest, but significant mutator effect in proofreading-proficient dnaQ+ strains [13]. This mutator effect likely reflects a slightly different interaction of Hot with the proofreading subunit, leading to diminished capacity to proofread. Although in the present study we have not directly measured the Hot mutator effect in the dnaQ+ background, it is very likely that the P1 prophage is capable of a similar mutator effect under these conditions. The ability to confer this mutator effect is an interesting aspect of the P1 biology, which may be relevant to its presence. We have previously suggested that the hot gene was acquired by P1 not from its current host E. coli, but from a more distantly related enterobacterial host [9]. Thus, Hot protein may have been optimized to function in conjunction with the ε proofreading subunit of that organism in its stabilizing function. Nevertheless, the ability to induce a mutator effect in E. coli may contribute to its persistence in the phage, as small genomes can tolerate and effectively benefit from increased mutation rates [25].
Acknowledgments
We thank Malgosia Lobocka, Institute for Biochemistry and Biophysics, for stimulating discussions on the subject of P1 hot, and we thank Marilyn Diaz and Giang Nguyen, NIEHS, for helpful comments on the manuscript for this paper. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- 1.Kelman Z, O’Donnell M. DNA polymerase III holoenzyme: structure and function of a chromosomal replicating machine. Annu Rev Biochem. 1995;64:171–200. doi: 10.1146/annurev.bi.64.070195.001131. [DOI] [PubMed] [Google Scholar]
- 2.McHenry CS. Chromosomal replicases as asymmetric dimers: studies of subunit arrangement and functional consequences. Mol Microbiol. 2003;29:1157–1165. doi: 10.1046/j.1365-2958.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell M. Replisome architecture and dynamics in Escherichia coli. J Biol Chem. 2006;281:10653–10656. doi: 10.1074/jbc.R500028200. [DOI] [PubMed] [Google Scholar]
- 4.Slater SC, Lifsics MR, O’Donnell M, Maurer R. holE, the gene coding for the θ subunit of DNA polymerase III of Escherichia coli: characterization of a holE mutant and comparison with a dnaQ (ε-subunit) mutant. J Bacteriol. 1994;176:815–821. doi: 10.1128/jb.176.3.815-821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taft-Benz SA, Schaaper RM. The θ subunit of Escherichia coli DNA Polymerase III: a role in stabilizing the ε proofreading subunit. J Bacteriol. 2004;186:2774–2780. doi: 10.1128/JB.186.9.2774-2780.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobocka MB, Rose DJ, Plunkett G, III, Rusin M, Samojedny A, Lehnherr H, Yarmolinsky MB, Blattner FR. Genome of bacteriophage P1. J Bacteriol. 2004;186:7032–7068. doi: 10.1128/JB.186.21.7032-7068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyrløv-Bendtsen J, Nilsson AS, Lehnherr H. Phylogenetic and functional analysis of the bacteriophage P1 single-stranded DNA binding protein. J Virol. 2002;76:9695–9701. doi: 10.1128/JVI.76.19.9695-9701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemonnier M, Ziegelin G, Reick T, Muñoz Gómez A, Diaz-Orejas R, Lanka E. Bacteriophage P1 Ban protein is a hexameric DNA helicase that interacts with and substitutes for Escherichia coli DnaB. Nucl Acids Res. 2003;31:3918–13918. doi: 10.1093/nar/gkg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chikova AK, Schaaper RM. The bacteriophage P1 hot gene product can substitute for the Escherichia coli DNA polymerase III θ subunit. J Bacteriol. 2005;187:5528–5536. doi: 10.1128/JB.187.16.5528-5536.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeRose EF, Kirby TW, Mueller GA, Chikova AK, Schaaper RM, London RE. Phage like it HOT: solution structure of the bacteriophage P1-encoded HOT protein, a homolog of the θ subunit of E. coli DNA polymerase III. Structure. 2004;12:2221–2231. doi: 10.1016/j.str.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Mueller GA, Kirby TW, DeRose EF, Li D, Schaaper RM, London RE. Nuclear magnetic resonance solution structure of the Escherichia coli DNA polymerase III θ subunit. J Bacteriol. 2005;187:7081–7089. doi: 10.1128/JB.187.20.7081-7089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirby TW, Harvey S, DeRose EF, Chalov S, Chikova AK, Perrino FW, Schaaper RM, London RE, Pedersen LC. Structure of the E. coli DNA polymerase III θ-Hot proofreading complex. J Biol Chem. 2006;281:38466–38471. doi: 10.1074/jbc.M606917200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chikova AK, Schaaper RM. Mutator and antimutator effects of the bacteriophage P1 hot gene product. J Bacteriol. 2006;188:5831–5838. doi: 10.1128/JB.00630-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehnherr H, Guidolin A, Arber W. Bacteriophage P1 gene 10 encodes a trans-activating factor required for late expression. J Bacteriol. 1991;173:6438–6445. doi: 10.1128/jb.173.20.6438-6445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehnherr H, Guidolin A, Arber W. Mutational analysis of the bacteriophage P1 late promoter sequence Ps. J Mol Biol. 1992;228:101–107. doi: 10.1016/0022-2836(92)90494-5. [DOI] [PubMed] [Google Scholar]
- 16.Singer M, Baker TA, Schnitzler G, Deischel SM, Goel M, Dove W, Jaacks KJ, Grossman AD, Erickson JW, Gross CA. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaaper RM, Danforth BN, Glickman BW. Rapid repeated cloning of mutant lac repressor genes. Gene. 1985;39:181–189. doi: 10.1016/0378-1119(85)90312-9. [DOI] [PubMed] [Google Scholar]
- 18.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLenigan MP, Kulaeva OI, Ennis DG, Levine AS, Woodgate R. The bacteriophage P1 HumD protein is a functional homolog of the prokaryotic UmuD′-like protein and facilitates SOS mutagenesis in Escherichia coli. J Bacteriol. 1999;181:7005–7013. doi: 10.1128/jb.181.22.7005-7013.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taft-Benz SA, Schaaper RM. Mutational analysis of the 3′→5′ proofreading exonuclease of Escherichia coli DNA polymerase III. Nucl Acids Res. 1998;26:4005–4011. doi: 10.1093/nar/26.17.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen AM, Lehnherr H, Wang X, Mobley V, Jin DJ. Escherichia coli SspA is a transcription activator for bacteriophage P1 late genes. Mol Microbiol. 2003;48:1621–1631. doi: 10.1046/j.1365-2958.2003.03533.x. [DOI] [PubMed] [Google Scholar]
- 22.Foster PL, Marinus MG. Levels of epsilon, an essential replication subunit of Escherichia coli DNA polymerase III, are controlled by heat shock proteins. J Bacteriol. 1992;174:7509–7516. doi: 10.1128/jb.174.23.7509-7516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McHenry CS, Kornberg A. DNA Polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1977;252:6478–6484. [PubMed] [Google Scholar]
- 24.Maki H, Maki S, Kornberg A. DNA Polymerase III holoenzyme of Escherichia coli. IV. The holoenzyme is an asymmetric dimer with twin active sites. J Biol Chem. 1988;263:6570–6578. [PubMed] [Google Scholar]
- 25.Drake JW. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci USA. 1991;88:7160–7164. doi: 10.1073/pnas.88.16.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]