Abstract
Only within the last two decades has the adult mammalian brain been recognized for its ability to generate new nerve cells and other neural structures and in essence to rewire itself. Although hippocampal structures have received the greatest scrutiny, other sites, including the cerebral cortex, also display this potential. Such processes remain active in the aging brain, although to a lesser degree. Two of the factors known to induce neurogenesis are environmental enrichment and physical activity. Gonadal hormones, however, also play crucial roles. Androgens and estrogens are both required for the preservation of cognitive function during aging and apparently help counteract the risk of Alzheimer’s disease. One overlooked threat to hormonal adequacy that requires close examination is the abundance of environmental endocrine-disrupting chemicals that interfere with gonadal function. They come in the form of estrogenic mimics, androgen mimics, anti-estrogens, anti-androgens, and in a variety of other guises. Because our brains are in continuous transition throughout the lifespan, responding both to environmental circumstances and to changing levels of gonadal steroids, endocrine-disrupting chemicals possess the potential to impair neurogenesis, and represent a hazard for the preservation of cognitive function during the later stages of the life cycle.
Keywords: Aging, Alzheimer’s disease, Androgens, Cognitive function, Endocrine disruption, Estrogens, Neurogenesis, Plasticity, Stroke, Synaptogenesis, Testosterone
INTRODUCTION
Until about two decades ago, the trajectory of brain aging seemed to advance along an indelible course. Our view of It was trapped in the dogma that once the brain received its alloted quota of nerve cells, its destiny was frozen. After that, the passage of time eroded our allotment steadily and irrevocably. Unlike our muscles, the prevailing mantra proclaimed, Use it and Lose it. No appeal; it was a sentence of life without parole. Nothing we could do would stem its inexorable advance. It was a disheartening prospect because no other organ system determines our identity and abilities and personality so absolutely.
Now we understand that aging offers a wider choice of transitions than we had appreciated in the past. It is not a fixed state. Like other complex biological processes, it is subject to modification at many steps as it unfolds. We did not grasp the possibility of modification because of the nearly universal belief that little existed. This belief was overturned by research during the last two decades that has fostered a radical reconstruction of our thinking. We now appreciate that the adult brain, rather than stalled into existence as a passive monolith, is instead a convergence of possibilities. We now know that some brain areas are capable of generating new neurons, and other brain elements such as dendritic spines, even during advanced age. We also are beginning to grasp the biological and environmental factors that underlie these activities. One of these, gonadal hormones, provides the main thrust of this article.
Gonadal hormones are largely (though not wholly) responsible for sexual differentiation of the reproductive organs and tissues. They also govern sexual differentiation of the brain during early development. The overt functional outcomes of this selective organization are sexually dimorphic behaviors, usually clear in the case of reproductive function but often quite subtle in the case of cognitive function. We understand these developmental events in considerable but not complete detail. In rough outline, the basic brain structure is female, and does not depend directly on gonadal steroids, while male characteristics arise during gestation when genetic males begin producing testosterone. The genetic mechanisms underlying sexual differentiation during development are being clarified by contemporary research. For example, Jeays-Ward et al (2004) describe the role of the signaling molecule Wnt4 in both male and female gonadal development. Another perspective is offered by Davies and Wilkinson (2006), who describe possible mechanisms by which genes located on sex chromosomes may help mold sexually dimorphic neurobehavioral development through processes distinct from those governed by gonadal hormones.
Our comprehension of how gonadal hormones modulate neurogenesis in the adult brain is much dimmer. This commentary will attempt to (1) tersely summarize our current and expanding appreciation of this process, and (2) join that knowledge to what we presently grasp about the potential for endocrine disruption by environmental chemicals.
For convenience, I will use the term, neurogenesis, to describe the formation of new neurons and of neuronal elements such as dendritic spines. The literature has become so overwhelming in size, with hundreds of publications appearing each month, that only a superficial review can be accommodated within the limited scope ot this article.
NEUROGENESIS IN THE ADULT BRAIN
More than 50 years ago, the psychologist Donald Hebb (Hebb, 1949) proposed that the brain generated new connections (“cell assemblies”) as biological representations of phenomena such as learning and memory. His model described a series of events that altered the responsivenes of existing neuronal networks. He did not envision a supporting architecture in the form of new cells and synapses, only a strengthening of those already in existence. It was a conceptual scheme bound by the prevailing view that the brain, once it attained maturity, had also attained its destined, static, population of nerve cells. That doctrine is often ascribed to anatomists such as Ramon y Cajal, who also wrote that, “It is for the science of the future to change, if possible, this harsh decree.”
Cajal’s harsh decree had been questioned as early as the 1960s (Altman, 1962), and clearly demonstrated to be false in songbirds who, so to speak, regrow certain brain structures each breeding season, (Nottebohm, 2002; Brainard and Doupe, 2002). It generally maintained its grip until the 1990s. By then, emerging studies had begun to promote a markedly different view of the brain’s inherent plasticity (Gross, 2000). They demonstrated the presence of neuronal precursors in the adult brain and the occurrence of neurogenesis—the production of new nerve cells. In many surprising ways, the process seemed to recapitulate those observed in the developing brain, including the integration of these newly-formed cells into the functional groupings of existing cell circuits. It cements the two poles of the lifespan into a common array of mechanisms. These studies also documented the dynamics of synapse and spine formation, ceaseless activities that continue even during advanced age (Kempermann et al, 2003). They showed that the aging brain still retained the ability to compensate for the progressive decline in dendritic spine density observed in anatomical studies (Coleman and Flood, 1987; Duan et al, 2003).
Especially during the past decade, a torrent of research has depicted the adult brain as a bustling real estate market in which old neurons are traded in for new ones that then move into the abandoned sites and in which entirely new collections are erected as well. They are presumed to develop from the population of multipotent neural precursors, arising from stem cells, that inhabit many areas of the adult mammalian brain. These newborn cells seem to be remarkably versatile. They can migrate to other brain regions, even those not normally hospitable to neurogenesis; they can blend into the existing circuitry; they can perform the required functions required by their new habitat; and they can differentiate into neuronal cell types dictated by the local microenvironment (e.g., Emsley, 2005). The process occurs in a series of steps:
1) Proliferation of neural stem-like cells (progenitors)
2) Progenitor cell commitment to neuronal phenotype (precursors)
3) Differentiation into immature neuron with specific properties
4) Migration to final location
5) Growth of axon, dendrites, synaptic connections
6) Maturation and integration into functional groups
This new perspective on brain plasticity has fueled a reappraisal of the inherent capabilities of the aging brain. One product of that reapprisal is the generation of potential strategies for overcoming or compensating for the diminution of cognitive function that accompanies aging.
Location, location
Much of our current appreciation of the plasticity of adult mammalian brains arose from observations in the subventricular zone (SVZ), the olfactory bulb (OB), and the dentate gyrus (DG) subregions of the hippocampus. The hippocampus is an attractive target for research because of its role in learning and memory formation and in disorders, such as Alzheimer’s disease, in which memory dysfunction is a major diagnostic index. Like the cells they replace, some of the newly-generated cells are transients rather than permanent residents. A significant fraction of newly generated neuronal cells in the DG and SVZ undergo programmed cell death. But as many as 9,000 or more new neuronal cells, or 0.1% of the granule cell population, are generated daily in the adult rat DG and embed themselves in it (Taupin, 2006). Of even greater relevance, Kempermann et al (2004) offer further evidence that these newly recruited cells persist in their new settings.
Even with the the critical role of the hippocampus in neurobehavioral function, were adult neurogenesis to be confined to that structure alone, it would prove of limited therapeutic potential. But it is now evident that it may be fostered in other brain areas as well. Zhao et al (2003), for example, provided the first experimental evidence, in mice, that such mechanisms may also operate in the adult substantia nigra, the structure where pronounced cell loss is presumed to be responsible for the clinical manifestations of Parkinson’s disease. Their results suggested that dopamine neurons are constantly turned over, although they die and are replaced at a low rate. Since then, a series of publications (e.g., Van Kampen et al, 2005 ) has confirmed the potency of dopamine as a stimulator of precursor cell proliferation in the rodent SVZ. Even more convincing, research by Freundlieb et al (2006) in aged primates has demonstrated a subventricular dopaminergic projection system from the substantia nigra pars compacta to the SVZ that suggests the possibility of using endogenous neural precursor cells to treat aged primates like ourselves. In these animal studies, a significant portion of the new neurons are integrated into the striatum. And Yoshimi et al (2005), in an examination of autopsy tissue from Parkinson’s disease patients, saw evidence of neurogenesis in nigral areas, a response to injury seen in other brain areas and disorders such as stroke.
Once the possibility of neurogenesis became tangible, questions arose about how it applied to parts of the brain, such as the cerebral cortex, that underlie the most complex behaviors. Here the data may be less plentiful and convincing, but Arlotta et al (2003) and Chen et al (2004) showed that neurogenesis may be induced in the adult mouse neocortex, an area in which it normally is not observed. They hold out the possibility that endogenous multipotent precursors in such areas might be manipulated in situ to replace lost or damaged neurons, even for neurodegenerative disorders such as motor neuron diseases.
Aging is a key determinant of the pace and adequacy of neurogenesis. Although new neurons are seen to occur throughout adulthood, they decline with age in DG, SVZ, and OB (Kuhn et al., 1996; Taupin, 2005). Several factors may explain such an age-related decline: depletion of multipotent precursors, a change in precursor cell properties, or a change in the properties of the internal brain environment. Physical exercise is an effective restorative, it seems. Old mice (19 months of age) given access to running wheels (van Praag et al, 2005), although showing fewer new nerve cells than their young (3 months of age) counterparts, produced considerably more new neurons than their sedentary controls. They also performed better on a water maze task.
Other kinds of manipulations are also encouraging about the potential of neurogenesis in the cerebral cortex. One way in which the modifiability of the adult brain is demonstrable besides its response to physical activity is in its response to environmental enrichment. Environmental enrichment has been known for five decades to nurture early brain development. Kempermann et al (2002) observed a dramatic increase in new neurons in mice 10 months of age living in a typical enriched environment for rodents (playmates and toys) for 10 months. Moreover, their performance on a battery of behavioral assays also exceeded those of the controls housed under standard conditions. Based on administration of the thymidine analog bromodeoxyuridine (BrdU), a marker of DNA synthesis that labels proliferating cells and their progeny, they interpreted their results as a demonstration that prolonged exposure to an enriched environment promotes survival of newly formed neurons.
Kozorovitskiy et al (2005), basing their methods on those shown to induce synaptogenesis in rodent brains, studied marmosets in three situations: one group lived in standard laboratory cages, the other two inhabited enrichment settings in which the subjects had access to a variety of objects to manipulate. Even a period as short as a month in a complex environment promoted longer and more complex dendritic trees and led to increased spine density in both hippocampus and prefrontal cortex. As in rodents, the primate brain, even in adulthood, retains the mechanisms, although not the magnitude, of plasticity characteristic of the developing brain.
Neurogenesis does not mean that we are replacing old circuits with new ones. If we did so on a massive scale, we might obliterate declarative and other long-term memory functions. A more likely explanation is that continuing replenishment of dysfunctional cells or cellular components is essential for maintaining function. In some respects, the process is analogous to the trimming of nerve cells during early development.
Does Neurogenesis Occur In Human Adults?
Evidence for neurogenesis in adult rodents has by now assumed massive proportions. Questions persist, however, about its occurrence in humans. Au and Fishell (2006) are among the skeptics who argue that the evidence, at least as it pertains to the adult neocortex, is far from convincing. Their argument is countered by a variety of sources. One comes from experiments in nonhuman primates, such as noted above in Freundlieb et al (2006). Several other experimenters have offered evidence from nonhuman primates. For example, Bedard et al (2002), based on BrdU labeling, found evidence for the production of new neurons in the striatum of adult squirrel monkeys. A useful summation of this and accompanying evidence, including newly-generated neurons in adult monkeys, was presented by Gould and Gross (2002). Most neuroscientists would find it surprising if phenomena unearthed in nonhuman primate brains were not applicable to human brains.
Another source of human evidence comes from brains of humans who have suffered strokes. Jin et al (2006) detected newly-formed neurons identified by endogenous-cell-proliferation and neuronal-lineage markers in the cortical ischemic penumbra of patients with stroke. Carmichael’s (2006) review of post-stroke functional recovery treats the possible mechanisms by which new growth might occur. He chooses, as one example, axonal sprouting in nonhuman primates subjected to stroke (Dancause et al, 2005; Dancause, 2006) that connects premotor cortex to primary sensory cortex, far from the site of injury. One of the signals he cites as a means of recruiting migrating neuroblasts to the area of injury is the cytokine erythropoietin (EPO). Post-lesion neurogenesis, such as exemplified by the publications above, is an extremely active area of research that is providing increasingly persuasive evidence that even the elderly human brain retains the fundamental substrates for neurogenesis.
Among the most intriguing possibilities are those revealed by studies showing that training can alter the amount of cortical geography devoted to specifice skllls. Draganski et al (2004) trained subjects for three months to learn a classic three-ball cascade juggling routine. Voxel-based morphometry, by tracing regional changes in gray matter, demonstrated enlarged bilateral expansion in the mid-temporal area and in the left posterior intraparietal sulcus between the original and the following scans, after they had become skilled performers. A later scan, three months later, after a period during which they had not practiced and no longer possessed those skills, showed that the enlargement had regressed. In career musicians, however, who continue to practice assiduously for years, such changes, as in string players, persist (Munte et al, 2002).
ROLE OF GONADAL HORMONES. THE ENDOCRINE SYSTEM AS A TARGET
As we became increasingly cognizant of the latent structural plasticity of the mature brain, we also began to recognize the potent influence wielded by gonadal steroid hormones on how this process is molded. Aging, neurogenesis, and gonadal hormones are tightly entwined. Gonadal hormones are not confined to reproductive functions and organs. They modulate and control both brain sexual differentiation during development and brain and behavioral function during adulthood. Developmental actions are termed “organizational” because they determine how brain structures vary with sex. Functional effects during adulthood, such as reproductive behaviors, are labeled as “activational” because they are linked to contemporaneous homonal state. Both organizational and activational effects are sexually dimophic and expressed in many subtle ways (Weiss, 2002a; Breedlove and Jordan, 2001). The hippocampus became a compellng target for investigators as it dawned on neuroscientists that hormones drove its ability to synthesize dendritic spines and synapses, and that such manifestations of brain plasticity during adulthood could account in part for learning and memory phenomena—Hebb redux.
Estrogens
One of the earliest indications of the role of estrogens came from studies of synaptogensis during the rodent estrus cycle. Woolley et al (1990) counted dendritic spines in the CA1 region of the hippocampus from female rats at different points in the estrus cycle, whose length is 4–5 days. To their surprise, the number varied with estrus cycle stage, peaking during proestrus. Although synaptogenesis had been observed earlier in the arcuate nucleus of the hypothalamus (Olmos et al, 1989), which is a structure important for reproductive behavior, it could be explained as a phenomenon correlated with the reproductive cycle. The hippocampal data provoked surprise because they came from a structure in which memories were assumed to be consolidated and stored. Synaptic turnovers of as much as 30% occurring in a day confounded the investigators. In further pursuit of the role of estrogen in synaptogenesis, Woolley et al (1992) removed the ovaries from adult females and treated one group with estrogen supplements. These females showed increased spine density in the CA1 compared with controls. She concluded that variations in estrogen levels during the estrous cycle underlay variations in dendritic spine formation.
In summary, these experiments showed that estrogens administered to ovariectomized rats produce the following effects:
Increases in dendritic spine density
Increases in frequency of presynaptic terminals
Increases in multiple spine innervations
Increases in multiple synaptic boutons
These observations have been confirmed and amplified in both primates and laboratory rodents (Leranth et al 2000; Leranth et al 2002; Hao et al 2003). Madeira et al (2001) correlated spine density with estrogen levels, which increase during the proestrus phase, and decrease during the diestrus phase. With direct administration of estradiol, the response is astonishingly rapid. MacLusky et al (2005a) observed a striking rise in synapse numbers 30 min after estradiol injection even when the vehicle was oil and was administered subcutaneously, a mode that slows release into the blood. Under physiological conditions, it is likely that synaptogenesis is initiated as soon as estradiol penetrates into the brain. Furthermore, spine synapse proliferation seems not to require high concentrations of estradiol in normal females; endogenous stores are adequate. In fact, Tanapat et al (2005) found that low doses of estradiol enhanced proliferation while high doses did not; U-shaped or inverted U-shaped functions are a common finding in endocrinology (Welshons et al, 2006).
Cooke and Wooley (2005), in their review of these relationships, also noted evidence that, during the period of elevated synaptogenesis, spatial navigation performance improved, followed by diminished performance when synaptogenesis waned. One explanation for this sequence, they speculated, derives from female exploration patterns. During proestrus, they wander over a large area to find willing males. Once fertilized, they confine their movements to a more restricted area.
The functional significance of estrogenic modulation of brain connectivity has generated considerable speculation. Much of that speculation is centered on aging and neurodegenerative disorders, particularly Alzheimer’s disease. The brain area luring the most research, the hippocampus, is also impaired in other neurodegenerative disorders (e.g., Parkinson’s disease and Huntington’s disease). In all of these disorders, diminished numbers of dendritic spines are observed. Such findings have generated speculation about and research on estrogen supplements. The current literature presents some puzzles that will be discussed in a later section of this article.
Androgens
Because of its role in shaping brain development and in the initial observations on adult brain plasticity, estrogen has dominated investigations coupling gonadal steroids and neurogenesis. The other major branch of gonadal hormones, androgens, has only recently begun to attract the curiosity of investigators (MacLusky et al, 2006; Parducz et al, 2006). One reason, as with estrogens, is their apparent influence on cognitive function, mood, and neurodegenerative disorders, Alzheimer’s disease in particular. Another compelling reason is their marked decline with age, in both sexes, which parallels rising deficits in the endpoints noted above. Now there are hints in the literature that arrestng the decline of androgen levels may reduce the risks of AD.
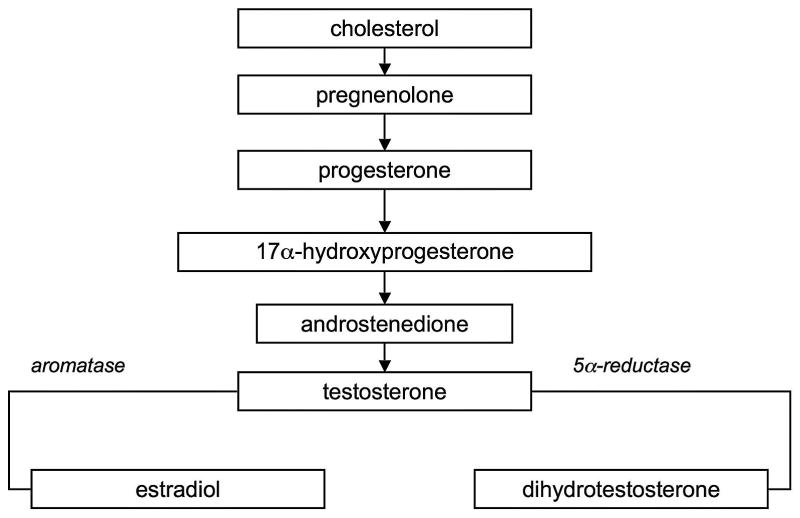
As shown in Figure 1, and as noted by MacLusky et al (2006), androgens in various forms serve as substrates for the formation of a number of biologically active agents in brain (e.g., see Hojo et al, 2003). For brain development, the conversion of testosterone to estradiol via the aromatase enzyme determines male anatomical features in rodents. In other tissues, testosterone is converted by 5α-reductase to dihydrotestosterone (DHT), which exceeds testostereone in potency in a variety of assays, and which determines differentiation of the male genitalia. The adrenal secretes dihydroepiandosterone (DHEA) which acts on peripheral tissues such as the mammary gland and prostrate. DHEA begins to fall steeply during middle age (Labrie et al 2001, 2005). Such changes evoke questions about how measures of reduced androgen function such as diminished circulating free testosterone, might promote Alzheimer’s disease (Hogervorst et al, 2004; Moffat et al, 2004). Data indicating that β-amyloid increases with testosterone deprivation supports such speculations (Almeida et al, 2004). A further intriguing complication arises from the apparent ability of the brain itself to synthesize gonadal hormones, as in Figure 1 (Mukai et al, 2006) and the reduction of this ability with aging and Alzheimer’s disease (Schumacher et al, 2003).
Figure 1.
Elements of steroid synthesis in adult male hippocampus (based on Hojo et al, 2004; Janowsky, 2006).
Leranth et al (2003) in rats and in monkeys (2004), spurred by apparent parallels between the actions of estrogren and androgens on dendritic spine density, investigated this possibility in orchidectomized rats and monkeys. In accord with their hypothesis, testes removal induced a steep reduction of spine synaptic density in CA1. Both testosterone itself and DHT restored spine density to control levels. Estradiol failed to do so; it was administered to test whether testosterone might act via aromatase conversion because DHT is not aromatized. The absence of an estradiol effect indicated that the hippocampal milieu is sexually dimorphic (Leranth et al, 2002). That is, males and females respond differently to estradiol. But of even more consequence was the demonstration that androgens can initiate the formation of dendritic spine synapses. Because of the reduction in synaptic density in hippocampus and prefrontal cortex associated with aging, and the parallel loss of memory function, such a finding suggests that androgen supplements may be useful in restoring performance (cf., Cherrier et al, 2003; Gray et al, 2005; Janowsky, 2006a,b).
Perhaps equally surprising, both testosterone and DHT also stimulated spine synapse formation in ovariectomized females. This finding is consistent with some reported effects of testosterone supplements in postmenopausal women. The underlying mechanisms are presently uncertain; MacLusky et al (2006) point to a varety of possibilities that might be exploited clinically. DHEA, considered a “weak” androgen, also increases spine synaptic density (Parducz et al, 2006) but as shown by Labrie (2005) and others, can be aromatized to estradiol.
Cognitive function and performance
The essential role of gonadal hormones for complex cognitive performance is firmly grounded in a swelling literature. Its growth attests to questions and possibilities aroused by its connection with aging. The motivation is no mystery: the relentless demographic crush besetting advanced industrial societies. The vanguard of that movement has already created massive problems in Europe and Japan. As it advances, a surge of neurodegerative disease trails in its wake.
The literature tells us that the waning levels of gonadal hormones attending aging also bring on waning levels of cognitive performance. This conjunction inspires hope as well as despair. It offers the prospect of arresting or even reversing declining cognitive ability because hormone supplements can be administered or their actions modified. The androgen literature offers less ambiguity for interventions than the estrogen literature.
Testosterone levels in men diminish, decade by decade, accompanied by a rise in sex hormone binding globulin (SHBG), which increases with age (Hijazi and Cunningham, 2005) and which reduces the free, bioavailable fraction of testosterone. In healthy men, the decline is gradual; by their seventies, their testosterone levels are about 40% below those found in men in their twenties. Although not wholly unambiguous, the literature largely supports the view that lower testosterone in older men is correlated with lower cognitive performance on tests of verbal and spatial memory (Moffat et al., 2002; Janowsky, 2006a,b). Figure 2 is based on Yaffe et al (2002), who found that free, rather than total testosterone, correlated with performance in a sample of older men (mean of 73 yrs old).
Figure 2.
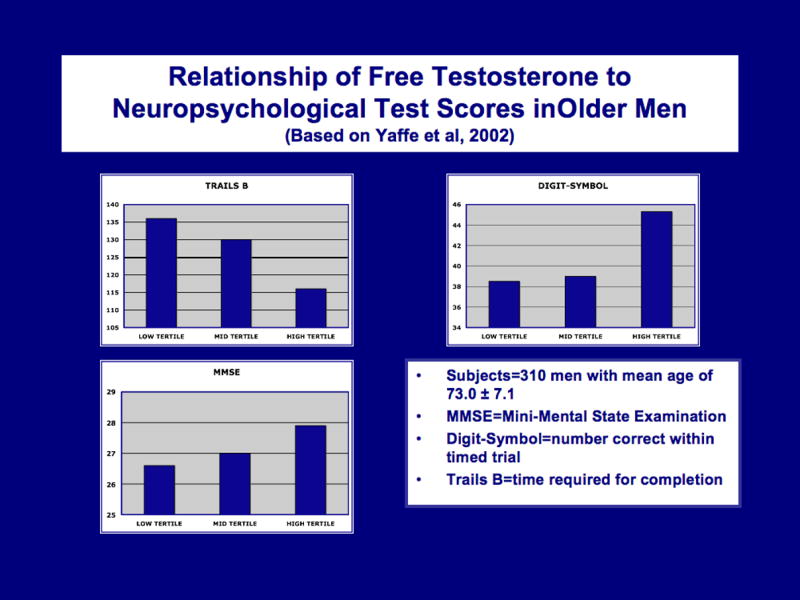
Relationship of free testosterone to neuropsychological test scores in older men (based on Yaffe et al, 2002). Higher levels are associated with better performance.
Scores on both short-term (working) and long-term (declarative) memory tests decline with aging. Providing testosterone or DHT supplements impoves spatial memory in both hypogonadal men (Cherrier et al., 2003) as well as older men (Gray et al., 2005).
Another source of data relating testosterone to cognitive function comes from studies of men with metastatic prostate cancer, who often are treated by testosterone deprivation (Janowsky, 2006a). One reported outcome of such treatment is diminished performance on tests of attention and verbal memory, an intriguing result because these functions are presumed to rely on the hippocampus. Lower testosterone levels are also seen as a risk factor for subsequent diagnosis of Alzheimer’s disease. The elevated risk may be associated, as some reports suggest, with increased deposition of ß-amyloid.
To summarize:
Memory function in older men (and women) is positively correlated with testosterone levels.
Testosterone supplements improve spatial cognition and working memmory in older men but not young men.
Lower testosterone levels predict a higher risk of Alzheimer’s disease.
Low testosterone level is correlated with increased ß-amyloid deposition.
Androgen deprivation for prostate cancer is correlated with lowered verbal memory performance.
The connection between estrogen and cognitive function in humans is more ambiguous. In animals, however, the connection is transparent. Nonhuman primates, like humans, suffer declines in cognitive performance during advanced age, a decline correlated more closely with a decline in synaptic spine density than with actual cell loss. In aged female monkeys, which, like perimenopausal women have undergone endocrine senescence, estrogen treatment increased CA1 spine density (Leranth et al, 2002; Hao et al 2003). Moreover, in ovariectomized monkeys, both spine number and delayed-response performance were enhanced in response to estrogen treatment (Rapp et al, 2003). Also in monkeys, Tang et al (2004) ovariectomized young monkeys and then administered either estradiol or vehicle intramuscularly. Here, their particular region of interest was area 46 of the prefrontal cortex, where they found a 55% increase in spine density in layer 1. Layer 1 is of particular interest for questions about neuroplasticity and endocrine status because synaptic density in that region declines markedly with age in monkeys, a decline correlated with performance on a delayed non-matching to sample task (Peters et al, 1998).
Along with the findings of Woolley et al (1990) and Gould et al (1990), which also showed increases in synaptic spine density in CA1 associated with estrogen, such data suggested that estrogen replacement therapy in postmenopausal women might counteract the decline in cognitive performance seen with aging. One way to evaluate such a hypothesis would be to test it in women who have undergone surgical removal of the uterus and ovaries for non-malignant disorders. In one study of the hypothesis (Phillips and Sherwin, 1992), women who received monthly intramuscular injections of estradiol following surgery performed better on tests of short-term and long-term verbal memory than women who received placebo injections. Other controlled trials have failed to observe any benefits (e.g., Janowsky et al, 2000; Binder et al, 2001). In these investigations, women with a mean age of 69 (Janowsky et al, 2000) and 81 (Binder et al, 2001) were supplied with conjugated equine estrogen (CEE) or placebo. CEE contains at least ten estrogenic compounds (Zhao and Brinton, 2006), so one possible explanation for the difference in findings is a pharmacokinetic one. The CEE was administered orally, and its major metabolite is estrone, which does not easily penetrate the blood-brain barrier. In addition, it promotes hepatic production of SHBG, which reduces the levels of free estrogen and testosterone. The parenteral route bypasses the liver.
Another possibility, now supported by data from several studies, is that early hormonal intervention tends to produce protective effects, while late intervention, several or more years beyond menopause, is either without effect or, in fact, tends to produce adverse outcomes. These were seen in the disappointing results of the The Women’s Health Initiative Memory Study (WHIMS). It was conducted as a component of the Women’s Health Initiative (WHI) program and examined the effects of estrogen plus progestin, estrogen alone in a group of hysterectomized women, or placebo on the risk of dementia and on mild cognitive impairment and global cognitive abilities. Instead of the anticipated positive effects of estrogen, women in the estrogen-progestin group proved to be at higher risk for dementia than those in the placebo group. No differences appeared in the estrogen-only group (Espeland et al 2004; Shumaker et al 2004).
A number of explanations might be given for these unexpected results compared to those of the earlier trials. One, noted earlier was the reliance on oral CEE rather than parenteral estradiol. A second, more subtle and intriguing one, is the time at which hormone supplementation was initiated. Both the animal and epidemiological literature indicate that the time at which replacement therapy begins may determine whether it confers beneficial effects. In one study, women who began treatment early after menopause experienced a lower risk of AD than those who had initiated treatment later (Zandi et al 2002). At enrollement in the WHIMS, the participants were between 65 and 79 years of age (with a mean of 73) and were followed for five years. They may have been too far past menopause, as noted by Sherwin (2007); they had been postmenopausal for approximately 21 years following their presumed spontaneous menopause, at an average age of 51 years. Moreover, many of the participants suffered from hypertension (40%), with a higher prevalence in the CEE group, and vascular disease often coexists with Alzheimer’s disease.
Both Brinton (2005) and Sherwin (2006) speak of a “window of opportunity” coincident with the early phases of menopause during which hormone replacement protects against cognitive decline and AD. The mechanisms remain to be determined, but, in essence, it appears that the brain at menopause still retains an environment hospitable to neuronal preservation and, given what is now known of hippocampal function and memory, neurogenesis as well. Brinton (2005) views these results as support for the principle she terms the “Healthy Cell Bias of Estrogen Action.” Estrogens as post-menopause treatments, in already compromised organisms, offer no benefits and, in fact, seem to exacerbate the risk of AD. She points to both in vivo and in vitro laboratory studies for supporting evidence, bolstered by the clinical trials referenced above and by others. Hao et al (2006) offer similar arguments to explain the WHIMS results.
A definitive resolution of these questions seems to recede further and further with each new study. MacLennan et al (2006) reported that women who initiated hormone therapy early (before age 56) performed better than late initiators and women who had never adopted hormone therapy. Erickson et al (2005) and Boccardi et al ( 2006), on the basis of voxel-based morphometry, reported that HRT appears to spare gray matter in some cortical regions, an effect magnified by longer durations of therapy, at least up to 10 years. Beyond that duration, both anatomical and functional indices appear to deteriorate. Lord et al (2006) report a similar effect of duration on hippocampal volume. Solving the puzzle of efficacy requires disentangling the contributions of the chemical form in which estrogen is administered, the mode of administration, accompanying agents such as progestins, age at initiation, and duration of therapy.
ENDOCRINE DISRUPTORS
Endocrine-disrupting chemicals (EDCs) are commonly viewed as compounds in the environment that degrade the ability of of endogenous hormones to function in their natural biological roles. Disruptor science first materialized in response to depleted wildlife populations and phenomena such as the puzzling behavior of female-female pairings in birds in the absence of male volunteers (Colborn et al, 1996). As the explanation for these observations narrowed to environmental contaminants, it pointed to imbalances in endocrine function as the responsible mechanism. It argued that many chemical contaminants in the environment interfered with the biological function of hormones by blocking, mimicking, displacing, distorting, or acting through a variety of other pathways such as epigenetic mechanisms to subvert their natural roles. It inevitably began to register that humans occupy the same planet as animals and that our bodies must obey the same biological rules. Their implications for public health stirred concern because, unlike many conventional toxic chemicals, they were demonstrated to act at environmentally relevant doses.
The full repercussions of human exposure to the immense range of environmental chemicals able to modify endocrine function are still beyond the domain of most health policy venues. They have eluded clear regulatory policy because they tend to display complex dose-response, nonmonotonic functions (typical in endocrinology) and involve cellular mechanisms that often act through multiple signaling pathways (Gore et al, 2006).
One of the repercussions arises from the powerful influence that endocrine disruptors exert on brain development. It owes that status to the huge volume of information published during the past three decades on the vulnerability of the developing brain to neurotoxic chemicals such as metals and to how brain development is molded by hormones. It prepared scientists to entertain the possibility that endocrine-disrupting environmental chemicals could also interfere with developmental processes. Many publications have explored these implications (e.g., Colborn, 2004; Schantz and Widholm, 2001; Weiss, 2002a; Melcagni and Panzica, 2006). The effects of these chemicals on aging, in contrast, are a tabula rasa. Yet they cannot be ignored.
Environmental endocrine disruptors intersect with our concerns about brain aging because we are now aware of the powerful influence that gonadal hormones, in particular, exert on neurogenesis and synaptogenesis. These processes, in fact, are marks of a healthy adult brain. Table 1 contains examples of documented envionmental estrogens and anti-estrogens; it is far from complete. Table 2 is a partial list of environmental anti-androgens. So far, there is hardly any literature connecting endocrine disruptors and how they might influence neurogenesis in the adult brain. One intriguing pair of contributions from MacLusky et al (2005a,b) illustrates the possibilities. They showed that fairly modest exposures to the plasticizer Bisphenol A, at levels less than the USEPA reference dose, inhibited the elaboration of synaptic spines in ovariectomized female rats treated with estradiol. Bisphenol A, by conventional assays, would be considered a weak estrogen. Through enzyme induction, rather than by binding to the estrogen receptor, it enhances cell proliferation to produce estrogen-like results (Welshons et al, 2006). It is not an estrogen, that is, in the conventional sense, and, in fact, as shown by MacLusky et al (2005b), it can also counteract the effects of estrogens.
Table 1.
Examples of Environmental Estrogens and anti-estrogens
|
|
Table 2.
Examples of Antiandrogenic Chemicals in The Environment
|
A similar inhibitory effect on the long term potentiation induced by estradiol also followed administration of Bisphenol A (Mukai et al, 2006). In a study of the antiandrogenic plasticizer diethylhexyl phthalate (DEHP), Andrade et al (2006) found it to be a potent aromatase inhibitor; Figure 1 shows how such a mechanism can interfere with the protective actions of testosterone on hippocampal neurogenesis.
Table 3 lists the kinds of products in which Bisphenol A is found. It also lists those containing phthalates, which are potent antiandrogens and which, at environmental levels, act to feminize infant boys exposed during pregnancy (Swan et al, 2005). It is inescapable, given the pervasive distribution of such chemicals, that the possibilities for endocrine disruptors acting on the aging brain, through their ability to interfere with gonadal hormones, deserve far more intense scrutiny than they have so far received.
Table 3.
Two Important Disruptors
|
|
The lists in tables 1 and 2 underscore an aspect of endocrine disruption that deserves far more recognition than it has received. The environment exposes us to a complex broth of chemicals whose constituents studied in isolation (typical of “mechanistic” studies) provide little information about how they act in combination. In their studies of environmental estrogens, Rajapakse et al (2002) found robust enhancement of steroid action when combiined, even at levels considerably below the individual no-effect values. Similarly, Gray et al (2006) showed that a combination of seven antiandrogenic chemicals of different chemical classes, assessed by their effects on anogenital distance (a marker of masculine development), showed remarkably linear dose additivity.
Although the role of gonadal hormones in neurogenesis adults is now firmly established, it is not now possible to claim a firm connection between endocrine-disrupting chemicals and effects on adult neurogenesis. The case has to be made indirectly, primarily because current disruptor research has focused so intensely on perinatal development for both neurobehavioral and reproductive function. It has to be acknowledged that so far the disruptor-neurogenesis connection is mostly a speculative one. At the same time, it would be difficult to argue against such a connection. What kind of case could be made for dismissing it?
EDCs are “weak;” that is, they produce relatively minor effects at environmental levels. Welshons et al (2006) effectively demolish this argument. They point out that the “weak” label is the result of insensitive and irrelevant assay procedures as well as the mistaken view that it is essentially an estrogen. And, even those that are “weak” comprise only one contribution to the total disruptor exposure.
No effects of EDCs have been seen so far in human populations. This is a misreading of the literature. For example, Swan (2006) and Hauser (2006), in their surveys of the links between environmental chemicals and fertility, offer convincing arguments that some currently used pesticides and other disruptors are significantly associated with reduced sperm viability by linking pesticide concentration in men's urine to results of semen analyses. Phthalate exposure during pregnancy is correlated with feminization of male offspring (Swan et al, 2005; Marsee et al, 2006) and with lowered testosterone concentrations (Main et al, 2005). Pan et al (2006) measured serum free testosterone values of workers exposed to dibutyl phthalate (DBP) and DEHP in a factory producing PVC flooring. They observed a significant reduction of testosterone in workers with higher levels of phthalate metabolites compared with unexposed workers. Data such as these demonstrate that environmental chemicals wih endocrine disruptor properties, especially those reflecting impaired androgenic function, have the potential to interfere with gonadal hormone-dependent neurogenesis. And, especially intriguing because it coincides with the release of the film, The Children of Men, an analysis of testosterone levels from a prospective cohort study in Massachusetts (Travison et al, 2007) concluded the following: “These results indicate that recent years have seen a substantial, and as yet unrecognized, age-independent population-level decrease in T in American men, potentially attributable to birth cohort differences or to health or environmental effects not captured in observed data.”
The neurogenesis characteristic of early development may be influenced by hormonal imbalances, but neurogenesis in adulthood is a different kind of process. In fact, however, the processes are equivalent in many respects. As Alvarez-Buylla and Lim (2004) observed, “It is now becoming clear that pieces of the embryonic developmental puzzle are retained for adult neurogenesis.” Taupin (2006) also noted that adult neurogenesis may emulate developmental processes. Although some fundamental differences, such as scale, are indisputable, as noted by Kempermann (2004), the similarities are still striking. They are even more striking from the standpoint of gonadal hormone action.
Epilogue
The contemporary view of the adult brain’s versatility in producing new growth presents neurotoxicology with new questions and challenges. No one would claim that the aging brain, even under the most promising circumstances, can blossom with the same profusion of cells and synapses that characterize it during the early phases of development. But even a superficially trivial expansion of new growth during adulthood may help diminish the inexorable decline in functional capacity that accompanies aging.
Figure 3 depicts the consequences of such a minor expansion. The diamonds represent estimates by Kety (1956) of the rate of decline in cerebral blood flow and oxygen consumption during the period from early adulthood to senescence. His model depicts a reduction of 20% in functional capacity by about age 68 years. The squares depict the effects of an acceleration of this process by 0.1% per year; here, a 20% reduction has been acheved by age 58 years. The triangles trace the outcome of slowing the decline in functional capacity by 0.1% per year. A functional decline of 20% is then delayed until 88 years of age. This is only a heuristic model, but it proves useful in arguing that what seem like trivial improvements can over time cumulate to exert significant consequences—a “tipping point,” as it were (Gladwell, 2000). In this instance, these consequences are far from trivial for a society confronting a population tilted toward senescence instead of youth. Yaffe (2003) described the outcome in stark terms: “More than 33% of women and 20% of men aged 65 and older will develop dementia during their lifetime. With the aging of the US population, the number of individuals with Alzheimer disease (AD) and other forms of dementia is expected to quadruple over the next 50 years.”
Figure 3.
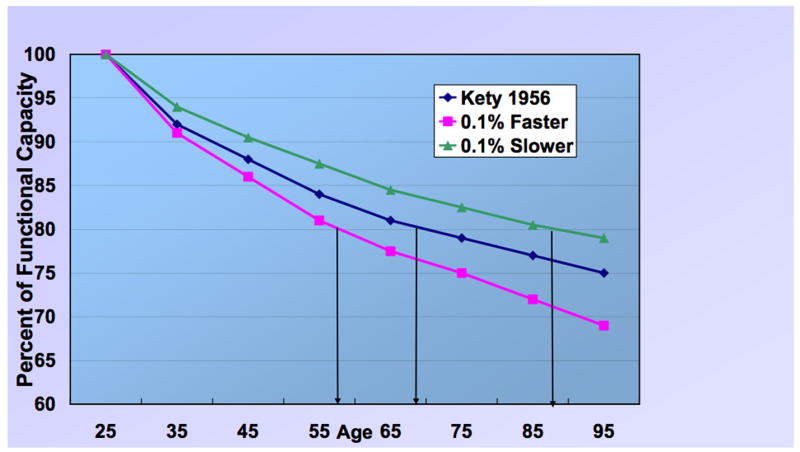
Different rates of decline with aging in CNS functional capacity based on cerebral blood flow and oxygen consumption (based on Kety, 1956). The diamonds represent estimates of the rate of decline in cerebral blood flow and oxygen consumption during the period from early adulthood to senescence. The squares depict the effects of an acceleration of this process by 0.1% per year. The triangles trace the outcome of slowing the decline in functional capacity by 0.1% per year.
Figure 4 diagrams the risk assessment challenges for brain aging generated by endocrine disruptors. The interconnected trio of aging, endogenous hormones, and neurogenesis embodies complications enough, as this commentary has attempted to demonstrate. Adding a fourth member to the ensemble, environmental contaminants, adds far more complications than a 25 % increase in personnel, a principle that both chamber music composers and rock groups have ingeniously exploited. The complications transcend the measures and mechanisms that ordinarily would be deployed to assay hormone levels because endocrine disruptors act in manifold ways. It can surely be argued that the endocrine system itself is increasingly susceptible to disruption as the organism ages. I submit that such a possibility should arouse the interest of neurotoxicologists; it is a research area that we should not abandon to endocrinologists. McEwen et al (2001) have a blunt message for us: “The brain is widely responsive to gonadal hormones.”
Figure 4.
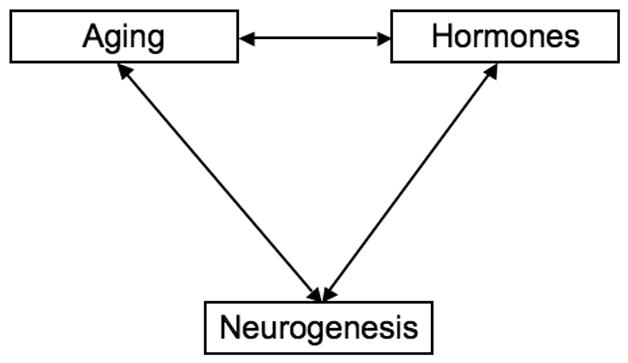
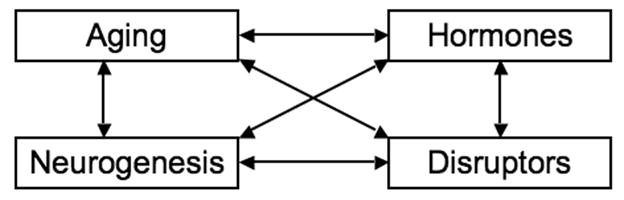
Interactions among aging, hormones, and neurogenesis are multiplied by the influence of endocrine disruptors.
The implications of that last statement present neurotoxicology and its regulatory translations with new dilemmas and opporunities. Exposure to endocrine disruptors such as pesticides spans the full life cycle (Weiss, 2000). In the past, we often tended to distinguish two realms of neurotoxicity based on chronological age. One centered on early development, the other on senescence. It is now profoundly clear that this distinction is arbitrary. Because of dramatic consequences such as those exemplified by fetal alcohol syndrome and congenital Minamata disease, this early segment of the lifespan has received the most intense scrutiny. Neurotoxicity is a lifetime issue, however, and our appreciation of its scope will suffer without extending our purview across the entire lifespan. Early development and aging are simply two narrow sectors from the arc of the lifespan, which itself is fundamentally a developmental journey. This is not to deny that early life events, whose influence is not evident at the time, may leave traces that only emerge decades later, when the patterns of vulnerability have changed (e.g., Weiss et al, 2002b). But the connection is not direct. The aging brain represents a mosaic of possibilities; some regions diminish, some even prosper. Finally, the legacy of earlier events is inevitably transformed by a cascade of intervening circumstances, such as stress, acting on this regrettable trek. For neurotoxicity is not simply a property of a designated chemical exposure, but is also a property dependent on the history and state of the host. Senescence offers its own contours of vulnerability and epitomizes a life stage not yet accorded its proper role in neurotoxicology’s domain. Although It is convenient to refer to different life periods as if they were tangible entities, we basically are viewing the same river from different vantage points.
This compressed and superficial survey of an explosively expanding research area merely highlights its connections with only one segment of neurotoxicology—endocrine disruption. We can be certain as well that other neurotoxic exposures during adulthood and beyond may exert effects on brain structure and function whose roots are planted in the inability of the brain to regrow and repair itself. One example is the work of Gilbert et al (2005). These investigators found that chronic lead exposure reduced neurogenesis in the dentate granule cell layer of the hippocampus. We have done little to pursue such questions, although we have acknowledged the reduced compensatory capacity of the aging brain.
But given the knowledge we’ve already achieved about how neurotoxicants act during the early phases of the lifespan, how can we resist asking the same kinds of questions about its late phases?
Acknowledgments
Preparation supported in part by grant ES013247 from NIEHS to Bernard Weiss and by NIEHS Center grant ES01247.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29:1071–1081. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): Non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227:185–192. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Magavi SS, Macklis JD. Molecular manipulation of neural precursors in situ: Induction of adult cortical neurogenesis. Exp Gerontol. 2003;38:173–182. doi: 10.1016/s0531-5565(02)00156-0. [DOI] [PubMed] [Google Scholar]
- Au E, Fishell G. Adult cortical neurogenesis: Nuanced, negligible or nonexistent? Nat Neurosci. 2006;9:1086–1088. doi: 10.1038/nn0906-1086. [DOI] [PubMed] [Google Scholar]
- Bedard A, Cossette M, Levesque M, Parent A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci Lett. 2002;328:213–216. doi: 10.1016/s0304-3940(02)00530-x. [DOI] [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM. Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas. 2001;38:137–146. doi: 10.1016/s0378-5122(00)00214-0. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Ghidoni R, Govoni S, Testa C, Benussi L, Bonetti M, Binetti G, Frisoni GB. Effects of hormone therapy on brain morphology of healthy postmenopausal women: A voxel-based morphometry study. Menopause. 2006;13:584–591. doi: 10.1097/01.gme.0000196811.88505.10. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Jordan CL. The increasingly plastic, hormone-responsive adult brain. Proc Natl Acad Sci U S A. 2001;98:2956–2957. doi: 10.1073/pnas.071054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: Evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: Making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci U S A. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Craft S, Matsumoto AH. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: A preliminary report. J Androl. 2003;24:568–576. doi: 10.1002/j.1939-4640.2003.tb02708.x. [DOI] [PubMed] [Google Scholar]
- Colborn T, Dumanoski D, Myers JM. Our stolen future. New York: Dutton; 1996. [Google Scholar]
- Colborn T. Neurodevelopment and endocrine disruption. Environ Health Perspect. 2004;112:944–949. doi: 10.1289/ehp.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and alzheimer's disease. Neurobiol Aging. 1987;8:521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Dancause N. Vicarious function of remote cortex following stroke: Recent evidence from human and animal studies. Neuroscientist. 2006;12:489–499. doi: 10.1177/1073858406292782. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W, Wilkinson LS. It is not all hormones: Alternative explanations for sexual differentiation of the brain. Brain Res. 2006;1126:36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Raz N, Korol DL, Scalf P, Webb A, Cohen NJ, McAuley E, Kramer AF. Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiol Aging. 2005;26:1205–1213. doi: 10.1016/j.neurobiolaging.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J Women's Health Initiative Memory Study. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's health initiative memory study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Freundlieb N, Francois C, Tande D, Oertel WH, Hirsch EC, Hoglinger GU. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Kelly ME, Samsam TE, Goodman JH. Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol Sci. 2005;86:365–374. doi: 10.1093/toxsci/kfi156. [DOI] [PubMed] [Google Scholar]
- Gladwell M. The tipping point. New York: Little Brown; 2000. [Google Scholar]
- Gore AC, Heindel JJ, Zoeller RT. Endocrine disruption for endocrinologists (and others) Endocrinology. 2006;147:S1–3. doi: 10.1210/en.2005-1367. [DOI] [PubMed] [Google Scholar]
- Gould E, Gross CG. Neurogenesis in adult mammals: Some progress and problems. J Neurosci. 2002;22:619–623. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT, Guillette L. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. discussion 105–8. [DOI] [PubMed] [Google Scholar]
- Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, Dzekov C, Sinha-Hikim I, Bhasin S. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J Clin Endocrinol Metab. 2005;90:3838–3846. doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: Death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R. The environment and male fertility: Recent research on emerging chemicals and semen quality. Semin Reprod Med. 2006;24:156–167. doi: 10.1055/s-2006-944422. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. New York: Wiley; 1949. [Google Scholar]
- Hijazi RA, Cunningham GR. Andropause: Is androgen replacement therapy indicated for the aging male? Annu Rev Med. 2005;56:117–137. doi: 10.1146/annurev.med.56.082103.104518. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for alzheimer's 2710 disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–1020. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. Thinking with your gonads: Testosterone and cognition. Trends Cogn Sci. 2006;10:77–82. doi: 10.1016/j.tics.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Dev Biol. 2004;276:431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kety SS. Human cerebral blood flow and oxygen consumption as related to aging. Res Publ Assoc Res Nerv Ment Dis. 1956;35:31–45. [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci U S A. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: Intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Prange-Kiel J, Frick KM, Horvath TL. Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cereb Cortex. 2004;14:503–510. doi: 10.1093/cercor/bhh012. [DOI] [PubMed] [Google Scholar]
- Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: Implications for parkinson's disease and memory. J Neurosci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: A possible window of opportunity effect. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.09.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: The REMEMBER pilot study. Menopause. 2006;13:28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect. 2005;113:675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138:957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: Stereological evaluation and golgi study. J Comp Neurol. 2001;432:329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, Andersson AM, Toppari J, Skakkebaek NE. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114:805–809. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: Implications for estrogen-induced synapse formation. Proc Natl Acad Sci U S A. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi RC, Panzica GC. Neuroactive steroids: Old players in a new game. Neuroscience. 2006;138:733–739. doi: 10.1016/j.neuroscience.2005.10.066. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Mukai H, Takata N, Ishii HT, Tanabe N, Hojo Y, Furukawa A, Kimoto T, Kawato S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: Synaptocrinology. Neuroscience. 2006;138:757–764. doi: 10.1016/j.neuroscience.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Munte TF, Altenmuller E, Jancke L. The musician's brain as a model of neuroplasticity. Nat Rev Neurosci. 2002;3:473–478. doi: 10.1038/nrn843. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57:737–749. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- Olmos G, Naftolin F, Perez J, Tranque PA, Garcia-Segura LM. Synaptic remodeling in the rat arcuate nucleus during the estrous cycle. Neuroscience. 1989;32:663–667. doi: 10.1016/0306-4522(89)90288-1. [DOI] [PubMed] [Google Scholar]
- Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): A cross-sectional study in china. Environ Health Perspect. 2006;114:1643–1648. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C. Synaptic remodeling induced by gonadal hormones: Neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8:671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Scholze M, Kortenkamp A. Deviation from additivity with estrogenic mixtures containing 4-nonylphenol and 4-tert-octylphenol detected in the E-SCREEN assay. Environ Sci Technol. 2004;38:6343–6352. doi: 10.1021/es049681e. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environ Health Perspect. 2001;109:1197–1206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's health initiative memory study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Swan SH. Does our environment affect our fertility? some examples to help reframe the question. Semin Reprod Med. 2006;24:142–146. doi: 10.1055/s-2006-944420. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL Study for Future Families Research Team. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Taupin P. Adult neurogenesis in mammals. Curr Opin Mol Ther. 2006;8:345–351. [PubMed] [Google Scholar]
- Taupin P. Consideration of adult neurogenesis from basic science to therapy. Med Sci Monit. 2005;11:LE16–17. [PubMed] [Google Scholar]
- Travison TG, Araujo AB, O'donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in american men. J Clin Endocrinol Metab. 2007;92:196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Robertson HA. A possible role for dopamine D3 receptor stimulation in the induction of neurogenesis in the adult rat substantia nigra. Neuroscience. 2005;136:381–386. doi: 10.1016/j.neuroscience.2005.07.054. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Sexually dimorphic nonreproductive behaviors as indicators of endocrine disruption. Environ Health Perspect. 2002;110(Suppl 3):387–391. doi: 10.1289/ehp.02110s3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Vulnerability to pesticide neurotoxicity is a lifetime issue. Neurotoxicology. 2000;21:67–73. [PubMed] [Google Scholar]
- Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ Health Perspect. 2002;110(Suppl 5):851–854. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K. Hormone therapy and the brain: Deja vu all over again? JAMA. 2003;289:2717–2719. doi: 10.1001/jama.289.20.2717. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- Yoshimi K, Ren YR, Seki T, Yamada M, Ooizumi H, Onodera M, Saito Y, Murayama S, Okano H, Mizuno Y, Mochizuki H. Possibility for neurogenesis in substantia nigra of parkinsonian brain. Ann Neurol. 2005;58:31–40. doi: 10.1002/ana.20506. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC Cache County Memory Study Investigators. Hormone replacement therapy and incidence of alzheimer disease in older women: The cache county study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (premarin) are protective against neurodegenerative insults: Implications for a composition of estrogen therapy to promote neuronal function and prevent alzheimer's disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]