Abstract
Background
The RD50 (exposure concentration producing a 50% respiratory rate decrease) test evaluates airborne chemicals for sensory irritation and has become an American Society for Testing and Materials (ASTM) standard method. Past studies reported good correlations (R2) between RD50s and the occupational exposure limits, particularly threshold limit values (TLVs).
Objective
The main purpose of this study was to examine the relationship between RD50s and human sensory irritation responses in a quantitative manner, particularly for chemicals that produce burning sensation of the eyes, nose, or throat, based on lowest observed adverse effect levels (LOAELs) reported for human subjects.
Methods
We compared RD50s with LOAELs and acute reference exposure levels (RELs). RELs, developed by the California Environmental Protection Agency’s Office of Environmental Health Hazard Assessment, represent a level at which no adverse effects are anticipated after exposure. We collected RD50s from the published literature and evaluated them for consistency with ASTM procedures. We identified LOAELs for human irritation and found 25 chemicals with a corresponding RD50 in mice.
Discussion
We found the relationship between RD50s and LOAELs as log RD50 = 1.16 (log LOAEL) + 0.77 with an R2 value of 0.80. This strong correlation supports the use of the RD50 in establishing exposure limits for the public. We further identified 16 chemical irritants with both RD50s and corresponding acute RELs, and calculated the relationship as log RD50 = 0.71 (log REL) + 2.55 with an R2 value of 0.71. This relationship could be used to identify health protective values for the public to prevent respiratory or sensory irritation.
Conclusion
Consequently, we believe that the RD50 has benefits for use in setting protective levels for the health of both workers and the general population.
Keywords: Alarie test, exposure levels, LOAEL, RD50, REL, sensory irritation, TLV
Although airborne chemicals can cause a number of harmful effects, the most common effect is sensory irritation (De Ceaurriz et al. 1981). Exposure to a sensory irritant may stimulate the trigeminal nerve endings and laryngeal receptors, eliciting any one or a combination of the following symptoms: burning sensation of the eyes, nose, or throat, as well as coughing sensations (Alarie et al. 2000). Sensory irritation is also the most common end point for occupational exposure levels (OELs). For one specific OEL measure, threshold limit values (TLVs) [developed by the American Conference of Governmental Industrial Hygienists (ACGIH 2006)] are calculated based on sensory or pulmonary irritation for > 50% of the compounds. Kane et al. (1979) reported that approximately two-thirds of the compounds for which they found a TLV acted as sensory irritants. A qualitative evaluation of sensory irritants indicated that sensory irritation responses in the mouse are predictive of responses in humans (Alarie 1973a).
In 1966, Alarie initially proposed the use of an animal test to evaluate the potency of airborne sensory irritants. The bioassay uses male Swiss-Webster mice to measure decreases in respiratory frequency resulting from exposure to a geometric series of concentrations of airborne irritants (Alarie 1966). The concentration inducing a 50% decrease in respiratory frequency is termed the RD50. From these measured RD50s, Alarie (1981b) ranked irritant potencies and found a good correlation (R2) between RD50s and TLVs. The Alarie test evolved over the years and was adopted in 1984 as a standard test by the American Society for Testing and Materials (ASTM 2004). The “RD50 test” or the “Standard Test Method for Estimating Sensory Irritancy of Airborne Chemicals” (ASTM 2004) quantitatively measures irritancy as indicated by the reflex inhibition of respiration in mice exposed to sensory irritants. For the test, four mice are first acclimatized to the chamber and are then simultaneously exposed to the airborne chemical. A sufficient number of groups are exposed to a geometric series of concentrations so that a concentration–response curve can be constructed from the analysis. The mice are placed in a body plethysmograph attached to an exposure chamber so that only the head is exposed to the test material. The plethysmographs are connected to pressure transducers, which sense changes created by inspiration and expiration. The amplified signals are transmitted to a polygraph recorder. The concentration of airborne irritant that produces an RD50 is determined from the concentration–response curve constructed from the various data points obtained with a series of concentrations.
Sensory irritation is a reflex reaction from stimulation of the trigeminal or laryngeal nerve endings (Boylstein et al. 1996). The sensory irritant response is mediated through binding to the trigeminal nerve receptors and appears to follow Michaelis-Menten receptor kinetics. Although the RD50 concentration has been described as “intolerable” to humans, as indicated in the ASTM standard, “the test method will detect irritation effects at concentrations far below those at which pathological changes are observed” (Alarie 2000; ASTM 2004). Further, as demonstrated by Barrow et al. (1986), pathologically detectable responses are expected only after prolonged repeated exposure.
RD50s are a basis, at least partially, for a number of OELs by ACGIH (ACGIH 2006). The calculation methodology is based on Kane et al. (1979), who evaluated data from 11 sensory irritants and concluded that a level one-hundredth of the RD50 would produce “minimal or no sensory irritation” in humans. The current suggestion of setting OELs at 0.03 RD50 comes from Alarie (1981a, 1981b), because 0.03 RD50 is halfway between 0.1 RD50 and 0.01 RD50 on a logarithmic scale. Alarie (1981a) reported a strong correlation (R2 = 0.89) between 0.03 RD50 and OELs for the 26 chemicals tested. Subsequently, both analyses, one using 41 chemicals (Alarie and Luo 1986) and most recently another using 89 chemicals (Schaper 1993), resulted in a lower but still strong correlation (R2 = 0.78). Although most of the applications of the RD50 have focused on OELs, Nielsen et al. (1995) found that protection against indoor sensory irritation effects could be achieved at a level of 0.025–0.25 of the OEL. Multiple studies show strong correlations between RD50s and OELs, supporting the continued use of the Alarie test for establishing OELs (Kane et al. 1979, 1980; Schaper 1993).
In this study we examined the relationship between RD50s and human sensory irritation responses in a quantitative manner, particularly for chemicals that produce burning sensation of the eyes, nose, or throat, based on lowest observed adverse effect levels (LOAELs) reported for human subjects. We also analyzed the relationship between RD50s and OELs for identified human sensory irritants. Finally, we evaluated the relationship between RD50s and acute reference exposure levels (RELs) developed to protect the public (Collins et al. 2004). RELs are defined as “[t]he concentration level at or below which no adverse health effects are anticipated for a specified exposure duration [1 hr for the acute RELs]. … RELs are based on the most sensitive, relevant, adverse health effect reported in the medical and toxicological literature.” A strong correlation between RD50s and LOAELs, TLVs, and acute RELs will support the use of RD50s in establishing guidance levels to protect the public from sensory irritants.
Methods
LOAELs versus RD50s
Using published toxicologic studies of human subjects exposed to sensory irritants, we identified human LOAELs. Criteria for selecting human LOAELs required that the studies describe mild irritating effects (Alexeeff et al. 2002) resulting from acute inhalation exposure. Published human studies on hazardous air pollutants (HAPs) served as the primary sources of information for these chemicals (Alexeeff et al. 2002). We searched PubMed (National Library of Medicine; http://www.ncbi.nlm.nih.gov/sites/entrez), Biosis (www.biosis.org/), Current Contents (http://scientific.thomson.com/products/ccc/), Toxline (National Library of Medicine; http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?TOXLINE), SciFinder Scholar (Chemical Abstracts Service; http://www.cas.org/support/scifi/sfsolutions/index.html), Oldmedline (http://www.nlm.nih.gov/databases/databases_oldmedline.html), Web of Science (http://scientific.thomson.com/products/wos), and Environmental Sciences and Pollution Management Databases (Cambridge Scientific Abstracts; http://www.csa.com/factsheets/envclust-set-c.php) to identify toxicologic studies published between 1970 and 2005 for all 189 HAPs. Search terms included the chemical name, the type of LOAEL effects (e.g., irritation), route of exposure (inhalation), and exposure duration (acute). We also conducted online searches for additional non-HAP chemicals with an identified RD50. Further, we conducted manual searches from secondary sources through 2005. Five criteria were developed for inclusion of a study in the analysis: a) peer-reviewed and published, well-conducted industry-sponsored studies or doctoral dissertations; b) inhalation exposure; c) discrete acute exposure; d) available LOAEL for a mild adverse health effect; and e) the original research. For each human study analyzed, information about the chemical, exposure time, end-point category (eye and/or respiratory irritation), and LOAELs were recorded. If multiple mild responses were reported at various dose levels for the same chemical and exposure time, then the lowest adverse effect level was considered the LOAEL.
For RD50s, we first reviewed references identified from the database developed by Schaper (1993). We identified additional studies from Alarie et al. (2000). We also searched the scientific literature during the period 1992–2005 to identify newer published studies containing RD50s. For each identified study, we recorded information on the chemical, exposure time, species, and RD50. We reviewed the methodology used to attain each RD50 for consistency with current ASTM methods (ASTM 2004); for this reason, we included studies with mice, but excluded studies with rats in this analysis.
In cases where both RD50s and human LOAELs were available for the same chemical, we log transformed and fit the data with a linear relationship using Microsoft Office Excel 2003 (Microsoft, Redmond, WA) and SAS version 9.1 (SAS Institute Inc., Cary, NC) for Windows. This procedure was similar to previous RD50 comparisons (e.g., Alarie 1981b). When we found multiple LOAELs or RD50s for a single chemical, we considered each reported value in the analysis. Sensitivity analyses were conducted by evaluating the correlation generated from the regression of LOAELs with RD50 value data sets, which varied by exposure time, or strain tested. We also conducted subanalyses using upper and lower respiratory tract effects.
RELs versus RD50s
As reported by Collins et al. (2004), the California Environmental Protection Agency (EPA) has developed 51 acute inhalation RELs. We evaluateds these RELs to identify those based on eye or respiratory irritation end points in humans, and compared with RD50s. Using Microsoft Office Excel 2003 (Microsoft) and SAS version 9.1 (SAS) for Windows, we log transformed and fit the data with a linear relationship.
TLVs versus RD50s
For all RD50s used in the above analyses, we identified TLVs from ACGIH (2006). The TLVs included time-weighted averages, short-term exposure limits and ceilings. If the documentation reported more than one TLV value, we used the lowest, more protective value. A third comparison between RD50s and TLVs of identified human irritants, based on identification of a human LOAEL for irritation, was conducted using log-transformed data, fit with a linear relationship, and analyzed with Microsoft Office Excel 2003 (Microsoft) and SAS version 9.1 (SAS) for Windows.
Results
LOAELs versus RD50s
From our search, we identified 25 chemicals with 72 human acute irritation LOAELs from 49 studies (Table 1). The adverse effects, exposure times, and information reflecting the quality of the study (e.g., placebo-control, blinding, subject selection, subject characteristics, exposure design, and data reporting) are indicated in Table 1. For the 25 chemicals identified, 63 RD50s were found in mice (Table 2). The RD50s were based on seven mouse strains and exposure times ranging from 5 to 180 min.
Table 1.
LOAELs for human sensory irritation for each study found in the literature.
| Compound | LOAEL (ppm) | Time (min) | No. of subjects | % Responsea | End pointb | Reference |
|---|---|---|---|---|---|---|
| Acetaldehyde | 7 | 5 | 27 | 0 | Eye irritation | Stephens et al. 1961 |
| 12 | 4 | 9 | Averagec | Bronchial hyperresponsiveness (L) | Myou et al. 1994d | |
| 50 | 15 | 12 | Majority | Eye irritation | Silverman et al. 1946 | |
| Acetone | 300 | 3–5 | 10 | Majority | Eye irritation | Nelson et al. 1943 |
| 800 | 20 | 27 | Average | Eye and weak nasal irritation | Dalton et al. 1997d | |
| 990 | 240 | 16 | 100 | Eye, mouth, and throat irritation | Seeber et al. 1992 | |
| 1,000 | 450 | 4 | 75 | Eye and throat irritation | Stewart et al.1975 | |
| Acrolein | 0.44 | NG | 10 | NG | Conjuctival and nasal irritation | Plotnikova 1960 |
| 0.5 | 5 | 36 | 20 | Eye irritation | Stephens et al. 1961 | |
| 0.6 | 5 | 16 | Average | Eye and nasal irritation | Hine et al. 1961 | |
| Allyl alcohol | 0.78 | 5 | 6 | Average | Slight nasal irritation | Dunlap et al. 1958 |
| Ammonia | 5 | 180 | 12 | 100 | Eye irritation | Sundblad et al. 2004d |
| 30 | 10 | 5 | 40 | Eye and nasal irritation | MacEwen and Vernot 1972 | |
| 50 | 30 | 16 | 44 | Eye and throat irritation | Verberk 1977 | |
| n-Butyl acetate | 200 | 3–5 | 10 | Majority | Throat irritation | Nelson et al. 1943 |
| n-Butanol | 25 | 3–5 | 10 | Majority | Eye, nasal, and throat irritation | Nelson et al. 1943 |
| Chlorine | 0.95 | 240 | 8 | Average | Forced vital capacity decrease (L) | Rotman et al. 1983d |
| 1 | 60 | 5 | Average | FEV1 decrease (L) | D’Alessandro et al. 1996d | |
| 1 | 480 | 29 | 100 | FEV1 decrease (L) | Anglen 1981d | |
| 1 | 120 | 29 | 100 | Urge to cough | Anglen 1981d | |
| 1 | 60 | 29 | 100 | Throat irritation | Anglen 1981d | |
| 2 | 60 | 8 | 100 | Urge to cough | Anglen 1981d | |
| 2 | 240 | 8 | 100 | Forced vital capacity decrease (L) | Anglen 1981d | |
| 2 | 120 | 8 | 75 | Throat irritation | Joosting and Verberk 1975 | |
| 2 | 60 | 8 | 25 | Nasal irritation | Joosting and Verberk 1975 | |
| 2 | 30 | 8 | 38 | Nasal and throat irritation | Joosting and Verberk 1975 | |
| Ethylacetate | 400 | 3–5 | 10 | Majority | Nasal and throat irritation | Nelson et al. 1943 |
| 402 | 240 | 16 | Average | Eye, nasal, and throat irritation | Seeber et al. 1992 | |
| Formaldehyde | 0.4 | 120 | 20 | Average | Rhinitis | Pazdrak et al. 1993d |
| 0.5 | 120 | 20 | 100 | Nasal irritation | Krakowiak et al. 1998d | |
| 0.69 | 480 | 109 | Average | Eye irritation | Horvath et al. 1988 | |
| 1 | 120 | 16 | 44 | Conjunctival irritation | Anderson and Molhave 1983 | |
| 1 | 6 | 27 | 100 | Eye irritation | Bender et al. 1983 | |
| 1 | 5 | 75 | 8 | Eye irritation | Stephens et al. 1961 | |
| 1 | 1.5 | 48 | Average | Nasal irritation | Weber-Tschopp et al. 1977 | |
| 1 | 90 | 18 | 84 | Eye, nasal, and throat irritation | Day et al. 1984 | |
| 1.01 | 180 | 19 | 21 | Eye irritation | Kulle et al. 1987d | |
| 2 | 10 | 15 | 53 | Eye irritation | Schachter et al. 1986 | |
| 2 | 40 | 15 | 60 | Eye irritation | Schachter et al. 1987 | |
| 3 | 180 | 9 | Average | Eye, nasal, and throat irritation | Sauder et al. 1986 | |
| 3 | 180 | 9 | Average | Eye, nasal, and throat irritation; FEV1 decrease (L) | Sauder et al. 1987 | |
| 3.01 | 20 | 24 | Average | Eye, nasal, and throat irritation | Green et al. 1989d | |
| Isophorone | 25 | 15 | 12 | NG | Eye, nasal, and throat irritation | Silverman et al. 1946 |
| Isopropyl acetate | 200 | 15 | 12 | Majority | Eye irritation | Silverman et al. 1946 |
| Isopropanol | 400 | 3–5 | 10 | Majority | Eye, nasal, and throat irritation | Nelson et al. 1943 |
| Methanol | 1025 | 240 | 1 | 100 | Eye irritation | Apol 1981 |
| Methyl ethyl ketone | 100 | 3–5 | 10 | Majority | Nasal and throat irritation | Nelson et al. 1943d |
| 200 | 240 | 19 | Average | Subclinical rhinitis | Muttray et al. 2002 | |
| Methyl isocyanate | 0.5 | 10 | 6 | 100 | Eye, nasal, and throat irritation | Smyth et al. 1970 |
| 1.75 | 1 | 8 | 38 | Nasal irritation | Smyth et al. 1970 | |
| 2 | 1 | 4 | 100 | Eye irritation | Kimmerle and Eben 1964 | |
| 2.5 | 120 | 7 | 57 | Nasal irritation | Pozzani and Carpenter 1963 | |
| Nitrogen dioxide | 1.5 | 180 | 15 | Average | Increased airway reactivity (L) | Frampton et al. 1991d |
| 2 | 60 | 18 | Average | Increased airway reactivity (L) | Mohsenin 1988d | |
| 2.5 | 120 | 16 | Average | Increased airway resistance (L) | Beil and Ulmer 1976 | |
| 5 | 120 | 16 | Average | Increased airway resistance (L) | von Nielding and Wagner 1977 | |
| n-Pentanol | 100 | 3–5 | 10 | Majority | Throat irritation | Nelson et al. 1943 |
| n-Pentyl acetate | 100 | 3–5 | 10 | Majority | Throat irritation | Nelson et al. 1943 |
| Styrene | 14.7 | 15 | 2 | 100 | Bronchospasm (L) | Moscato et al. 1987 |
| 216 | 20 | 3 | 3 | Nasal irritation | Stewart et al. 1968 | |
| 600 | 1 | NG | NG | Eye and nasal irritation | Wolf et al. 1956 | |
| 800 | 240 | 2 | 100 | Eye and throat irritation | Carpenter et al. 1944 | |
| Sulfur dioxide | 5 | 300 | 14 | Average | Increase in discomfort, irritation | Andersen et al. 1981 |
| Toluene | 100 | 360 | 16 | Average | Eye irritation | Anderson and Molhave 1983 |
| 100 | 390 | 24 | Average | Nasal and lower airway irritation | Baelum et al. 1990 | |
| 200 | 210 | 2 | 100 | Eye and throat irritation | Carpenter et al. 1944 | |
| 300 | 3–5 | 10 | Majority | Eye and throat irritation | Nelson et al. 1943 | |
| Toluene-2,4-diisocyanate | 0.01 | 900 | 15 | 7 | Increased airway resistance (L) | Baur 1985 |
| Triethylamine | 4.35 | 480 | 2 | 100 | Visual disturbances, discomfort | Akesson et al. 1986 |
| 8.22 | 240 | 2 | 100 | Visual disturbances, discomfort | Akesson et al. 1986 | |
| 11.6 | 60 | 2 | 100 | Visual disturbances, discomfort | Akesson et al. 1986 | |
| p-Xylene | 100 | 450 | 11 | 18 | Eye and respiratory irritation | Hake et al. 1981 |
Abbreviations: FEV1, forced expiratory volume in 1 sec; NG, not given. For some studies, multiple experiments were conducted with different exposure times or end points resulting in multiple LOAELs for the compounds.
Numerical values indicate the percent of subjects responding.
End points with (L) depict “Lower” respiratory end points; all others are “Upper” respiratory end points.
”Average” indicates that the response was a mean response.
Study was considered to be of higher quality due to study design (e.g., placebo-controlled, blinding, subject selection, subject characteristics, exposure conditions, and/or data reporting).
Table 2.
RD50s of male mice with their corresponding TLVsa and RELsb (ppm), along with the specific strain of mice used in the experiment and reference.
| Compound | RD50 (ppm) | Exposure time (min) | TLV (ppm) | REL (ppm) | RD50 strain | RD50 reference |
|---|---|---|---|---|---|---|
| Acetaldehyde | 2,845 | 10 | 25 | NA | SW | Steinhagen and Barrow 1984 |
| 2,932 | 10 | 25 | NA | B6C3F1 | Steinhagen and Barrow 1984 | |
| 4,946 | 10 | 25 | NA | SW | Kane et al. 1980 | |
| Acetone | 23,480 | 5 | 500 | NA | OF1 | de Ceaurriz et al. 1981 |
| 77,156 | 10 | 500 | NA | SW | Kane et al. 1980 | |
| Acrolein | 1.03 | 10 | 0.1 | 0.00009 | SW | Steinhagen and Barrow 1984 |
| 1.41 | 10 | 0.1 | 0.00009 | B6C3F1 | Steinhagen and Barrow 1984 | |
| 1.66 | 10 | 0.1 | 0.00009 | BALB/c | Muller and Greff 1984 | |
| 1.7 | 1 | 0.1 | 0.00009 | SW | Kane and Alarie 1977 | |
| 2.9 | 30 | 0.1 | 0.00009 | CF1 | Nielsen et al. 1984 | |
| Allyl alcohol | 1.6 | 5 | 0.5 | NA | OF1 | Muller and Greff 1984 |
| 2.5 | 30 | 0.5 | NA | ICR | James et al. 1987 | |
| 3.9 | 30 | 0.5 | NA | CF1 | Nielsen et al. 1984 | |
| Ammonia | 303 | 30 | 25 | 4.5 | SW | Barrow et al. 1978 |
| 789.6 | 10 | 25 | 4.5 | CF1 | Tomas et al. 1985 | |
| n-Butyl acetate | 730 | 5 | 150 | NA | OF1 | Muller and Greff 1984 |
| n-Butanol | 1,268 | 5 | 20 | NA | OF1 | de Ceaurriz et al. 1981 |
| 4,784 | 10 | 20 | NA | SW | Kane et al. 1980 | |
| 11,696 | 30 | 20 | NA | CF1 | Kristiansen et al. 1988 | |
| Chlorine | 3.50 | 120 | 0.5 | 0.07 | OF1 | Gagnaire et al. 1994 |
| 9.3 | 10 | 0.5 | 0.07 | SW | Barrow et al. 1977 | |
| 11.97 | 10 | 0.5 | 0.07 | BALB/c | Tomas et al. 1985 | |
| Ethylacetate | 580 | 5 | 400 | NA | OF1 | de Ceaurriz et al. 1981 |
| 614 | 10 | 400 | NA | SW | Kane et al. 1980 | |
| Formaldehyde | 3.1 | 10 | 0.3 | 0.076 | SW | Kane and Alarie 1977 |
| 4 | 10 | 0.3 | 0.076 | BALB/c | Nielsen et al. 1999 | |
| 4.9 | 10 | 0.3 | 0.076 | B6C3F1 | Chang et al. 1981 | |
| 5.3 | 5 | 0.3 | 0.076 | OF1 | de Ceaurriz et al. 1981 | |
| Isophorone | 27.8 | 5 | 5 | NA | OF1 | de Ceaurriz et al. 1981 |
| Isopropyl acetate | 4,259 | 5 | 100 | NA | OF1 | Muller and Greff 1984 |
| Isopropanol | 5,000 | 5 | 200 | 1.3 | OF1 | de Ceaurriz et al. 1981 |
| 17,693 | 10 | 200 | 1.3 | SW | Kane et al. 1980 | |
| Methanol | 25,222 | 5 | 200 | NA | OF1 | Muller and Greff 1984 |
| 41,514 | 10 | 200 | NA | SW | Kane et al. 1980 | |
| Methyl ethyl ketone | 9,000 | 10 | 200 | 4.5 | SW | Stone et al. 1981 |
| 10,745 | 5 | 200 | 4.5 | OF1 | de Ceaurriz et al. 1981 | |
| 31,426 | 30 | 200 | 4.5 | CF1 | Hansen et al. 1992 | |
| Methyl isocyanate | 1.3 | 90 | 0.02 | NA | SW | Ferguson et al. 1986 |
| 2.9 | 30 | 0.02 | NA | ICR | James et al. 1987 | |
| Nitrogen dioxide | 349 | 10 | 3 | 0.25 | SW | Alarie 1981c |
| Phenol | 166 | 5 | 1.5 | OF1 | de Ceaurriz et al. 1981 | |
| n-Pentanol | 4,039 | 10 | NA | NA | SW | Kane et al. 1980 |
| 5,933 | 5 | NA | NA | OF1 | Muller and Greff 1984 | |
| n-Pentyl acetate | 1,531 | 10 | 50 | NA | SW | Alarie 1981a |
| 1,562 | 5 | 50 | NA | OF1 | Muller and Greff 1984 | |
| Styrene | 156.3 | 3 | 20 | 5.1 | SW | Alarie 1973b |
| 586 | 5 | 20 | 5.1 | OF1 | de Ceaurriz et al. 1981 | |
| 980 | 10 | 20 | 5.1 | SW | Alarie 1981a | |
| Sulfur dioxide | 117 | 2 | 0.25 | SW | Alarie et al. 1981a | |
| Toluene | 3,373 | 5 | 50 | 9.8 | OF1 | de Ceaurriz et al. 1981 |
| 4,900 | 10 | 50 | 9.8 | SW | Dudek et al. 1990 | |
| 5,300 | 30 | 50 | 9.8 | SW | Nielsen and Alarie 1982 | |
| 2,4-Toluene | 0.24 | 40 | 0.005 | NA | OF1 | de Ceaurriz et al. 1981 |
| Diisocyanate | 0.39 | 30 | 0.005 | NA | SW | Barrow et al. 1978 |
| 0.78 | 180 | 0.005 | NA | SW | Sangha and Alarie 1979 | |
| Triethylamine | 156 | 15 | 1 | 0.68 | OF1 | Gagnaire et al. 1989 |
| 186 | 30 | 1 | 0.68 | CF1 | Nielsen and Yamagiwa 1989 | |
| p-Xylene | 1,325 | 5 | 100 | 5 | OF1 | Muller and Greff 1984 |
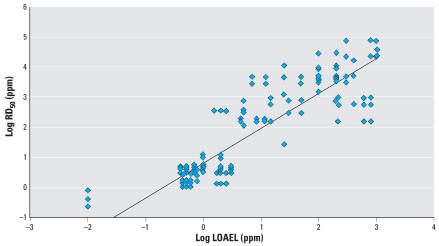
Figure 1 shows the correlation between RD50s and LOAELs for all RD50s identified in all strains of mice for the 25 chemicals, allowing for 198 comparisons. There is a strong overall correlation (R2 = 0.80) between RD50s and human irritation LOAELs. When we conducted the analysis for Swiss-Webster mice only (Table 3), we were able to include 75 data points for 19 compounds, and the correlation decreased slightly (R2 = 0.74). When we evaluated only the data for non–Swiss-Webster mice (Table 3), there was little change in the correlation (R2 = 0.83). We conducted several sub-analyses to consider the influence of the RD50 study exposure duration. As indicated in Table 3 there was little influence on the R2. Thus, according to this analysis, the strain of mouse tested does not appear to affect this evaluation substantially. The equations do not change significantly, and the correlation is still significant for all analyses, validating the inclusion criteria used. As indicated in Table 3, we also considered several subanalyses to address the influence of the human LOAEL variability. Specifically, we considered the issue of LOAEL sensitivity, the type of irritation end point, study quality, and the duration of exposure for the human LOAEL. The only significant effect on the correlation was observed when considering human irritation end points of the lower respiratory tract; the poor R2 appears to be attributed partly to the few number of data points (29) in the analysis.
Figure 1.
Linear least-squares regression analysis for log RD50 (for all mouse strains) vs. log LOAEL (human irritation end points) for 25 compounds, using 195 data points. Log RD50 = 1.16(log LOAEL) + 0.77; R 2 = 0.80.
Table 3.
Summary of linear least-squares regression analyses for various comparisons.
| Basic analyses | No. of compounds included | No. of data points included | Regression line | R2 value |
|---|---|---|---|---|
| Description of analysis | ||||
| All RD50s identified in all strains of mice vs. all human LOAELs identified (Figure 1) | 25 | 198 | logRD50 = 1.16(log LOAEL) + 0.77 | 0.82 |
| Evaluation using male mice and RELs set by OEHHA for airborne toxicants (Figure 2) | 16 | 37 | logRD50 = 0.71(log REL) + 2.55 | 0.71 |
| Evaluation using male mice and the TLV (Figure 3) | 24 | 61 | logRD50 = 0.86(log TLV) − 1.13 | 0.86 |
| Addressing issues of human LOAEL variabilities | ||||
| Evaluation using all RD50s identified in all strains of mice vs. the lowest human LOAEL for each compound | 25 | 58 | logRD50 = 1.13(log LOAEL) + 1.26 | 0.81 |
| Analysis for male mice log RD50 vs. log LOAEL using lowest RD50 values with the lowest LOAEL values | 25 | 25 | logRD50 = 1.01(log LOAEL) + 1.21 | 0.77 |
| Analysis for male mice log RD50 and human log LOAEL for lower respiratory end points | 5 | 29 | logRD50 = 1.06(log LOAEL) + 1.21 | 0.58 |
| Analysis for male mice log RD50 and human log LOAEL for upper respiratory end points | 23 | 166 | logRD50 = 1.22(log LOAEL) + 0.69 | 0.82 |
| Analysis for male mice log RD50 and human log LOAEL for higher quality human studies | 7 | 43 | logRD50 = 1.40(log LOAEL) + 0.98 | 0.82 |
| Analysis for male mice log RD50 and human log LOAEL for human studies not selected as higher quality | 25 | 155 | log RD50 = 1.16(log LOAEL) + 0.73 | 0.79 |
| Evaluating influence of mouse strain | ||||
| Evaluation using only Swiss-Webster mice and all human LOAEL values (Figure 2) | 19 | 75 | logRD50 = 1.12(log LOAEL) + 0.93 | 0.74 |
| Evaluation using all non–Swiss-Webster mice and all human LOAEL values (Figure 3) | 23 | 120 | logRD50 = 1.20(log LOAEL) + 0.73 | 0.83 |
| Evaluating changes in exposure duration | ||||
| Evaluation using male mice and human LOAEL values from exposures of ≤ 10 min | 16 | 67 | logRD50 = 1.27(log LOAEL) + 0.726 | 0.76 |
| Evaluation using male mice and human LOAEL values from exposures of > 10 min | 18 | 127 | logRD50 = 1.11(log LOAEL) + 0.838 | 0.80 |
| Evaluation using male mice and human LOAEL values from exposures of ≥ 60 min | 15 | 101 | logRD50 = 1.08(log LOAEL) + 0.89 | 0.80 |
| Log RD50 vs. log RD50 for RD50 values with time < 10 min | 16 | 44 | logRD50 = 1.04(log LOAEL) + 0.76 | 0.77 |
| Log LOAEL vs. Log RD50 for RD50 values with time > 10 min | 10 | 43 | logRD50 = 1.51(log LOAEL) + 0.56 | 0.87 |
| Log RD50 vs. log LOAEL for RD50 values with time equivalent to 10 min | 16 | 111 | logRD50 = 1.3(log LOAEL) + 0.78 | 0.80 |
| LogRD50 vs. log LOAEL for RD50 values at times not equivalent to 10 min | 22 | 86 | logRD50 = 1.09(log LOAEL) + 0.77 | 0.8 |
OEHHA, Office of Environmental Health Hazard Assessment.
RELs versus RD50s
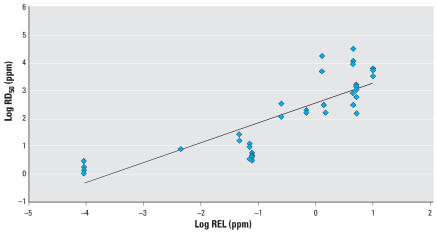
From the 51 California acute RELs, we identified 16 that had irritation as their end point and a corresponding RD50. Figure 2 indicates a good correlation (R2 = 0.71) between RD50s and RELs for 16 chemicals with 37 comparisons.
Figure 2.
Linear least-squares regression analysis for log RD50 (mice) vs. log REL (set by OEHHA for airborne toxicants) for 16 compounds. Log RD50 = 0.71(log REL) + 2.55; R 2 = 0.71.
TLVs versus RD50s
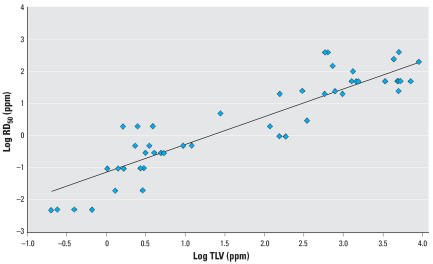
For the compounds identified with RD50s and LOAELs, 24 had a corresponding TLV. Figure 3 shows the correlation of TLVs to RD50s with an R2 value of 0.81. Thus, when focusing specifically on human irritants, the relationship between the TLV and RD50 remains strong.
Figure 3.
Linear least-squares regression analysis for log RD50 (male mice) vs. log TLV for 24 compounds (no TLV for n-pentanol). Log RD50 = 0.86(log TLV) – 1.13; R2 = 0.86.
Conclusions
The focus of this paper is on the applicability of RD50s for human health risk assessment. Exposure guidelines to protect workers and the public often focus on mild irritating signs or symptoms. For example, > 50% of the TLVs and > 60% of the California acute RELs based their end points on irritation (Collins et al. 2004). However, human studies from which to develop acute exposure guidance are not available for many of the hundreds of substances of concern, and therefore reliance on animal studies is necessary. The RD50 test method is appealing because it generates data rapidly, requires minimal animal use, is low in cost, and is validated, calibrated, and standardized. The method was computerized, adding to the reproducibility of the results (Alarie 1998, 2000; Vijayaraghavan et al. 1994). The availability of RD50s in male mice for 89 chemicals (Schaper 1993), and their correlation with OELs suggests potential applicability to air exposure guidelines for the public. The result of this analysis quantitatively supports the applicability of RD50s in setting exposure guidelines for the public and workers.
We found a strong correlation between RD50s and human LOAELs, TLVs, and California RELs. Focusing on human studies where the subjects developed eye or respiratory irritation responses, we observed a strong correlation (R2 = 0.80) between RD50s and LOAELs for 25 chemicals with irritating effects. The correlation remained close to 0.8 after conducting various subanalyses, indicating that the strains of mice or the RD50 exposure time does not substantially affect the correlation. Previously, Nielsen et al. (1995) proposed an indoor air guideline for the public between 0.025 and 0.25 times the OEL, similar to 0.0008 and 0.008 times the RD50. In our analysis, the RD50 to REL correlation can be expressed as REL = 0.00026 × RD501.4. Derived as follows:
 |
Exposure times in the human studies varied from 1 to 480 min, and a subanalysis looking specifically at the effect of the duration of exposure made no significant change to the correlation. Further, subanalyses using LOAELs more closely associated with either upper respiratory or lower respiratory effects did not make a significant change to the correlations. Although the variability in the response rate, interindividual sensitivity, and differences in human study design, as described in Table 1, would be expected to have reduced the correlation with the RD50, specific factors were not identified in our subanalyses. Thus, we conclude that the irritating symptoms in humans correlate well with the RD50s of animals irrespective of the specific acute exposure duration. These results not only support the use of the RD50 in setting guidelines for acutely irritating compounds, but also suggest that a concentration–time extrapolation for these effects appears unwarranted. This is consistent with the finding by Shusterman et al. (2006) that the human response to sensory irritants reached a plateau rapidly. Thus, the response appears to be influenced to a greater extent by the exposure concentration rather than the exposure time over the period of observation for most animal and human experiments considered in the present analysis, and over the periods of concern for the TLVs (15 min to 8 hr) and acute RELs (1 hr).
The results of this analysis are subject to several limitations. First, the number of available human studies limits the LOAEL data, and it is unlikely that human data will significantly increase in the future. The number of comparisons could increase as the numbers of RD50s increase for chemicals with human data. However, considering the robustness of the subanalyses, and the historical correlation of the RD50 to the TLV, a significant change in the RD50 to LOAEL correlation is unlikely after adding other sensory irritants in the analysis. Finally, we address issues raised by Bos et al. (1992, 2002, 2003).
First, Bos et al. (2003) claimed that the RD50–OEL correlation is expected because most OELs are based on animal data. Although many OELs are based on animal data, many are based on human data as well. Of the 24 substances we evaluated in our RD50–OEL correlation, the OEL for only one compound, n-pentyl acetate, relied on the RD50 for its derivation, which was based solely on animal data. The strong correlation between RD50s and human LOAELs also addresses this concern.
Second, Bos et al. (2002) reported the RD50s did not correlate well with histopathologic changes in the respiratory tract or with corrosivity, and therefore RD50s were inappropriate to evaluate respiratory tract irritation. However, the stated purpose of the ASTM standard is to evaluate sensory irritation potential, not histopathology or corrosivity. In our comparison of the RD50s with human irritation LOAELs, the correlation was strong with the inclusion of respiratory tract irritation end points in the analysis. Further, the risk assessment framework for occupational and public exposure levels addresses the concerns regarding the potential for other, more severe effects. In cases where other health effects occur at or below levels producing sensory irritation, exposure guidelines use the more sensitive adverse effect.
Third, Bos et al. (1992) raised concerns regarding the inconsistency of RD50s among strains and species. Although RD50s have been generated for various strains and species with varying test procedures, adhering to the ASTM standard method addresses this concern. Limiting the RD50 test to those conducted in mice, or Swiss-Webster mice, and limiting the exposure time keeps the test to a more standardized method, although intrastrain variability was not a cause for concern in our subanalyses. Finally, we addressed the concern regarding time–concentration response curves (Bos et al. 1992), with separate subanalyses based on exposure time. These analyses show that time did not appear to be a factor in our analyses. Our presumption is that if the study adheres adequately to the ASTM standard method, experimental exposure time plays a minor role. It is also worth pointing out that all of the figures comparing RD50s to LOAELs, RELs, and TLVs are plotted on a log–log plot because of the wide range of values. Because of the nature of log–log plots, the correlation is higher compared with the same correlation using a nonlogarithmic scale.
The applicability of the RD50 test to human health protection has been demonstrated in several analyses, but extrapolation of the test results to the general public would be improved with greater focus on the tail of the dose–response curve, to ensure protection of sensitive subpopulations. One solution would be for RD50 studies to report sufficient information to calculate a benchmark dose (BMD) value, and not focus solely on the specific RD50 value. A standardized BMD value could be calculated at the tail of the distribution, taking into account the slope of the dose–response curve. Alternatively, the test procedure could be refined to identify the “just detectable effect level,” which is approximately a 12% decrease in the respiratory rate (Alarie 1998). Although some work has been done in this area (Boylstein et al. 1996), additional information is needed to better understand the tail of the dose–response curve and to address any concerns for spurious results from low exposure concentrations. The reported just detectable effect level of 12% appears to be close to the no observed effect level of the procedure. Use of this response rate in risk assessment is consistent with the recommendation by the U.S. EPA (2007) that the BMD for a continuous response may be set on statistical criteria of distinguishability from the control value, as well as on grounds of anticipated biological significance. A major benefit of focusing on the just detectable effect level would be to reduce potential animal suffering, and possibly animal usage.
In conclusion, the RD50 test is a good starting point for setting exposure standards for acute airborne irritants. As noted by Alarie et al. (2000), the TLV may need to be < 0.03 RD50 to prevent other toxic effects. Consequently, the literature should be adequately evaluated to determine that sensory irritation is likely the most sensitive adverse effect. The application of RD50s appears most useful when qualitative data are available indicating sensory irritation as the most sensitive adverse effect, but quantitative human data are lacking. The RD50 has proven its usefulness with the ability to appropriately rank the potency of airborne chemicals as sensory irritants and help establish exposure limits. A strong correlation between RD50s and LOAELs provides further support for using RD50s in determining guidance levels to protect the general public from sensory irritants.
Footnotes
We thank K. Deng, M. Masuda, and S. Ackerman for their technical assistance in preparing the manuscript; and R. Howd, G. Krowech, and M. Schaper for their critical reading of the document and helpful comments.
The views expressed are those of the authors and do not necessarily represent those of the Office of Environmental Health Hazard Assessment, the California Environmental Protection Agency, or the State of California.
References
- ACGIH. TLVs and BEIs, Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists; 2006. [Google Scholar]
- Akesson B, Bengtsson M, Floren I. Visual disturbances after industrial triethylamine exposure. Int Arch Occup Environ Health. 1986;57(4):297–302. doi: 10.1007/BF00406184. [DOI] [PubMed] [Google Scholar]
- Alarie Y. Irritating properties of airborne materials to the upper respiratory tract. Arch Environ Health. 1966;13(4):433–449. doi: 10.1080/00039896.1966.10664593. [DOI] [PubMed] [Google Scholar]
- Alarie Y. Sensory irritation by airborne chemicals. CRC Crit Rev Toxicol. 1973a;2(3):299–363. doi: 10.3109/10408447309082020. [DOI] [PubMed] [Google Scholar]
- Alarie Y. Sensory irritation of the upper airways by airborne chemicals. Toxicol Appl Pharmacol. 1973b;24(2):279–297. doi: 10.1016/0041-008x(73)90148-8. [DOI] [PubMed] [Google Scholar]
- Alarie Y. Bioassay for evaluating the potency of airborne sensory irritants and predicting acceptable levels of exposure in man. Food Cosmet Toxicol. 1981a;19(5):623–626. doi: 10.1016/0015-6264(81)90513-7. [DOI] [PubMed] [Google Scholar]
- Alarie Y. Dose–response analysis in animal studies: prediction of human responses. Environ Health Perspect. 1981b;42:9–13. doi: 10.1289/ehp.81429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarie Y. Toxicological evaluation of airborne cemical irritants and allergens using respiratory reflex reactions. In: Leong BKJ, editor. Proceedings of the Inhalation Toxicology and Technology Symposium. Ann Arbor, MI: Ann Arbor Science Publications; 1981c. pp. 207–231. [Google Scholar]
- Alarie Y. Computer-based bioassay for evaluation of sensory irritation of airborne chemicals and its limit of detection. Arch Toxicol. 1998;72(5):277–282. doi: 10.1007/s002040050502. [DOI] [PubMed] [Google Scholar]
- Alarie Y. Computerized animal bioassay to evaluate the effects of airborne chemicals on the respiratory tract. In: Spengler JD, Samet JM, McCarthy JF, editors. Indoor Air Quality Handbook. New York: McGraw-Hill; 2000. pp. 24.1–24.25. [Google Scholar]
- Alarie Y, Luo JE. Sensory irritation by airborne chemicals: a basis to establish acceptable levels of exposure. In: Barrow CS, editor. Toxicology of the Nasal Passages. New York: Hemisphere Publishing; 1986. pp. 91–100. [Google Scholar]
- Alarie Y, Nielsen GD, Schaper MM. Animal bioassays for evaluation of indoor air quality. In: Spengler JD, Samet JM, McCarthy JF, editors. Indoor Air Quality Handbook. New York: McGraw-Hill; 2000. pp. 23.1–23.49. [Google Scholar]
- Alexeeff GV, Broadwin R, Liaw J, Dawson SV. Characterization of the LOAEL-to-NOAEL uncertainty factor for mild adverse effects from acute inhalation exposures. Regul Toxicol Pharmacol. 2002;36(1):96–105. doi: 10.1006/rtph.2002.1562. [DOI] [PubMed] [Google Scholar]
- Andersen I, Molhave L. Controlled human studies with formaldehyde. In: Gibson JE, editor. Formaldehyde Toxicity. Washington, DC: Hemisphere; 1983. pp. 154–164. [Google Scholar]
- Andersen I, Molhave L, Proctor DF. Human responses to controlled levels of combinations of sulfur dioxide and inert dust. Scand J Environ Health. 1981;7:1–7. doi: 10.5271/sjweh.2570. [DOI] [PubMed] [Google Scholar]
- Anglen DM. Sensory Response of Human Subjects to Chlorine in Air [PhD Thesis] Ann Arbor, MI: University of Michigan; 1981. [Google Scholar]
- Apol A. Health Hazard Evaluation Report. Seattle, WA: National Institute for Occupational Safety and Health, Hazard Evaluation and Technical Assistance Branch; 1981. [Google Scholar]
- ASTM. Standard Test Method for Estimating Sensory Irritancy of Airborne Chemicals. West Conshohocken, PA: ASTM International; 2004. [Google Scholar]
- Baelum J, Lundqvist GR, Molhave L, Andersen NT. Human response to varying concentrations of toluene. Int Arch Occup Environ Health. 1990;62(1):65–71. doi: 10.1007/BF00397850. [DOI] [PubMed] [Google Scholar]
- Barrow CS, Alarie Y, Stock MF. Sensory irritation and incapacitation evoked by thermal decomposition products of polymers and comparisons with known sensory irritants. Arch Environ Health. 1978;33(2):79–88. doi: 10.1080/00039896.1978.10667313. [DOI] [PubMed] [Google Scholar]
- Barrow CS, Alarie Y, Warrick JC, Stock MF. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch Environ Health. 1977;32:68–76. doi: 10.1080/00039896.1977.10667258. [DOI] [PubMed] [Google Scholar]
- Barrow CS, Buckley LA, James RA, Steinhagen WH, Chang JCF. Sensory irritation: studies on correlation to pathology, structure-activity, tolerance development, and prediction of species differences to nasal injury. In: Barrow CS, editor. Toxicology of Nasal Passages. Washington, DC: Hemisphere; 1986. pp. 101–122. [Google Scholar]
- Baur X. Isocyanate Hypersensitivity: Final Report to the International Isocyanate Insititute Project E-AB-19, File 10349. Munich, West Germany: University of Munich; 1985. [Google Scholar]
- Beil M, Ulmer WT. Effect of NO2 in workroom concentrations on respiratory mechanics and bronchial susceptibility to acetylcholine in normal persons [in German] Int Arch Occup Environ Health. 1976;38(1):31–44. doi: 10.1007/BF00378318. [DOI] [PubMed] [Google Scholar]
- Bender JR, Mullin LS, Graepel GJ, Wilson WE. Eye irritation response of humans to formaldehyde. Am Ind Hyg Assoc J. 1983;44(6):463–465. doi: 10.1080/15298668391405139. [DOI] [PubMed] [Google Scholar]
- Bos PM, Busschers M, Arts JH. Evaluation of the sensory irritation test (Alarie test) for the assessment of respiratory tract irritation. J Occup Environ Med. 2002;44(10):968–976. doi: 10.1097/00043764-200210000-00017. [DOI] [PubMed] [Google Scholar]
- Bos PM, Busschers M, Arts JH. Reply to: Sensory irritation testing. J Occup Environ Med. 2003;45(5):467. doi: 10.1097/01.jom.0000069240.06498.14. [DOI] [PubMed] [Google Scholar]
- Bos PM, Zwart A, Reuzel PG, Bragt PC. Evaluation of the sensory irritation test for the assessment of occupational health risk. Crit Rev Toxicol. 1992;21(6):423–450. doi: 10.3109/10408449209089882. [DOI] [PubMed] [Google Scholar]
- Boylstein LA, Luo J, Stock MF, Alarie Y. An attempt to define a just detectable effect for airborne chemicals on the respiratory tract in mice. Arch Toxicol. 70(9):567–578. doi: 10.1007/s002040050314. [DOI] [PubMed] [Google Scholar]
- Carpenter CP, Shaffer B, Weil CS, Smyth JHF. Studies on the inhalation of 1:3-butadiene; with a comparison of its narcotic effect with benzol, toluol, and styrene, and a note on the elimination of styrene by the human. J Ind Hyg Toxic. 1944;26(3):69–78. [Google Scholar]
- Chang JC, Steinhagen WH, Barrow CS. Effect of single or repeated formaldehyde exposure on minute volume of B6C3F1 mice and F-344 rats. Toxicol Appl Pharmacol. 1981;61(3):451–459. doi: 10.1016/0041-008x(81)90368-9. [DOI] [PubMed] [Google Scholar]
- Collins JF, Alexeeff GV, Lewis DC, Dodge DE, Marty MA, Parker TR, et al. Development of acute inhalation reference exposure levels (RELs) to protect the public from predictable excursions of airborne toxicants. J Appl Toxicol. 2004;24(2):155–166. doi: 10.1002/jat.967. [DOI] [PubMed] [Google Scholar]
- D’Alessandro A, Kuschner W, Wong H, Boushey HA, Blanc PD. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest. 1996;109(2):331–337. doi: 10.1378/chest.109.2.331. [DOI] [PubMed] [Google Scholar]
- Dalton P, Wysocki CJ, Brody MJ, Lawley HJ. Perceived odor, irritation, and health symptoms following short-term exposure to acetone. Am J Ind Med. 1997;31(5):558–569. doi: 10.1002/(sici)1097-0274(199705)31:5<558::aid-ajim10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Day JH, Lees RE, Clark RH, Pattee PL. Respiratory response to formaldehyde and off-gas of urea formaldehyde foam insulation. Can Med Assoc J. 1984;131(9):1061–1065. [PMC free article] [PubMed] [Google Scholar]
- de Ceaurriz JC, Micillino JC, Bonnet P, Guenier JP. Sensory irritation caused by various industrial airborne chemicals. Toxicol Lett. 1981;9(2):137–143. doi: 10.1016/0378-4274(81)90030-8. [DOI] [PubMed] [Google Scholar]
- Dudek B, Gralewicz K, Jakubowski M, Kostrzewski P, Sokal J. Neurobehavioral effects of experimental exposure to toluene, xylene and their mixture. Pol J Occup Med. 1990;3(1):109–116. [PubMed] [Google Scholar]
- Dunlap MK, Kodama JK, Wellington JS, Anderson HH, Hine CH. The toxicity of allyl alcohol. AMA Arch Ind Health. 1958;4:303–311. [PubMed] [Google Scholar]
- Ferguson JS, Schaper M, Stock MF, Weyel DA, Alarie Y. Sensory and pulmonary irritation with exposure to methyl isocyanate. Toxicol Appl Pharmacol. 1986;82(2):329–335. doi: 10.1016/0041-008x(86)90209-7. [DOI] [PubMed] [Google Scholar]
- Frampton MW, Morrow PE, Cox C, Gibb FR, Speers DM, Utell MJ. Effects of nitrogen dioxide exposure on pulmonary function and airway reactivity in normal humans. Am Rev Respir Dis. 1991;143(3):522–527. doi: 10.1164/ajrccm/143.3.522. [DOI] [PubMed] [Google Scholar]
- Gagnaire F, Azim S, Bonnet P, Hecht G, Hery M. Comparison of the sensory irritation response in mice to chlorine and nitrogen trichloride. J Appl Toxicol. 1994;14(6):405–409. doi: 10.1002/jat.2550140604. [DOI] [PubMed] [Google Scholar]
- Gagnaire F, Azim S, Bonnet P, Simon P, Guenier JP, de Ceaurriz J. Nasal irritation and pulmonary toxicity of aliphatic amines in mice. J Appl Toxicol. 1989;9(5):301–304. doi: 10.1002/jat.2550090504. [DOI] [PubMed] [Google Scholar]
- Green DJ, Bascom R, Healey EM, Hebel JR, Sauder LR, Kulle TJ. Acute pulmonary response in healthy, nonsmoking adults to inhalation of formaldehyde and carbon. J Toxicol Environ Health. 1989;28(3):261–275. doi: 10.1080/15287398909531347. [DOI] [PubMed] [Google Scholar]
- Hake CL, Stewart RD, Wu A, Graff SA, Forster HV, Keeler WH, et al. p-Xylene: Development of a Biologic Standard for the Industrial Worker by Breath Analysis. Cincinnati, OH: National Institute for Occupational Safety and Health; 1981. [Google Scholar]
- Hansen LF, Knudsen A, Nielsen GD. Sensory irritation effects of methyl ethyl ketone and its receptor activation mechanism. Pharmacol Toxicol. 1992;71(3 Pt 1):201–208. doi: 10.1111/j.1600-0773.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Hine CH, Meyers F, Ivanhoe F, Walker S, Takahashi GH. Proceedings of The Fifth Air Pollution Medical Research Conference: Symposium on Human Exposures to Air Pollutants 1961. Berkeley, CA: California State Department of Health; 1961. Simple tests of respiratory function and study of sensory response in human subjects exposed to respiratory tract irritants; pp. 20–38. [Google Scholar]
- Horvath EP, Jr, Anderson H, Jr, Pierce WE, Hanrahan L, Wendlick JD. Effects of formaldehyde on the mucous membranes and lungs. A study of an industrial population. JAMA. 1988;259(5):701–707. [PubMed] [Google Scholar]
- James JT, Buettner LC, Hsu SS. Sensory irritation of methylisocyanate vapor. J Appl Toxicol. 1987;7(2):147–148. doi: 10.1002/jat.2550070213. [DOI] [PubMed] [Google Scholar]
- Joosting PE, Verberk MM. Emergency population exposure: a methodological approach (with a report on a human experiment with chlorine). International Symposium on the Recent Advances in the Assessment of the Health Effects of Environmental Pollution; Paris, France. Luxembourg: Commission of the European Communities, WHO; 1975. 1974. pp. 2005–2029. [Google Scholar]
- Kane LE, Alarie Y. Sensory irritation to formaldehyde and acrolein during single and repeated exposures in mice. Am Ind Hyg Assoc J. 1977;38(10):509–522. doi: 10.1080/0002889778507665. [DOI] [PubMed] [Google Scholar]
- Kane LE, Barrow CS, Alarie Y. A short-term test to predict acceptable levels of exposure to airborne sensory irritants. Am Ind Hyg Assoc J. 1979;40:207–229. doi: 10.1080/15298667991429516. [DOI] [PubMed] [Google Scholar]
- Kane LE, Dombroske R, Alarie Y. Evaluation of sensory irritation from some common industrial solvents. Am Ind Hyg Assoc J. 1980;41(6):451–455. doi: 10.1080/15298668091425022. [DOI] [PubMed] [Google Scholar]
- Kimmerle G, Eben A. On the toxicity of methylisocyanate and its quantitative determination in the air [in German] Arch Toxicol. 1964;20:235–241. [PubMed] [Google Scholar]
- Krakowiak A, Gorski P, Pazdrak K, Ruta U. Airway response to formaldehyde inhalation in asthmatic subjects with suspected respiratory formaldehyde sensitization. Am J Ind Med. 1998;33(3):274–281. doi: 10.1002/(sici)1097-0274(199803)33:3<274::aid-ajim9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kristiansen U, Vinggaard AM, Nielsen GD. The effects of n-butanol vapour on respiratory rate and tidal volume. Arch Toxicol. 1988;61(3):229–236. doi: 10.1007/BF00316639. [DOI] [PubMed] [Google Scholar]
- Kulle TJ, Sauder LR, Hebel JR, Green DJ, Chatham MD. Formaldehyde dose-response in healthy nonsmokers. JAPCA. 1987;37(8):919–924. doi: 10.1080/08940630.1987.10466285. [DOI] [PubMed] [Google Scholar]
- MacEwen J, Vernot E. Annual Technical Report. Wright-Patterson Air Force Base, OH: Aerospace Medical Research Laboratory, Toxic Hazards Research Unit; 1972. [PubMed] [Google Scholar]
- Mohsenin V. Airway responses to 2.0 ppm nitrogen dioxide in normal subjects. Arch Environ Health. 1988;43(3):242–246. doi: 10.1080/00039896.1988.9934941. [DOI] [PubMed] [Google Scholar]
- Moscato G, Biscaldi G, Cottica D, Pugliese F, Candura S, Candura F. Occupational asthma due to styrene: two case reports. J Occup Med. 1987;29(12):957–960. [PubMed] [Google Scholar]
- Muller J, Greff G. Relation between the toxicity of molecules of industrial value and their physico-chemical properties: test of upper airway irritation applied to 4 chemical groups [in French] Food Chem Toxicol. 1984;22(8):661–664. doi: 10.1016/0278-6915(84)90276-x. [DOI] [PubMed] [Google Scholar]
- Muttray A, Jung D, Klimek L, Kreiner C. Effects of an external exposure to 200 ppm methyl ethyl ketone on nasal mucosa in healthy volunteers. Int Arch Occup Environ Health. 2002;75(3):197–200. doi: 10.1007/s00420-001-0291-3. [DOI] [PubMed] [Google Scholar]
- Myou S, Fujimura M, Nishi K, Matsuda M, Ohka T, Matsuda T. Potentiating effect of inhaled acetaldehyde on bronchial responsiveness to methacholine in asthmatic subjects. Thorax. 1994;49(7):644–648. doi: 10.1136/thx.49.7.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KW, Ege JJF, Morwick R, Woodman LE, Silverman L. Sensory response to certain industrial solvent vapors. J Ind Hyg Toxic. 1943;25(7):282–285. [Google Scholar]
- Nielsen GD, Alarie Y. Sensory irritation, pulmonary irritation, and respiratory stimulation by airborne benzene and alkylbenzenes: prediction of safe industrial exposure levels and correlation with their thermodynamic properties. Toxicol Appl Pharmacol. 1982;65(3):459–477. doi: 10.1016/0041-008x(82)90391-x. [DOI] [PubMed] [Google Scholar]
- Nielsen GD, Alarie Y, Poulsen OM, Nexo BA. Possible mechanisms for the respiratory tract effects of noncarcinogenic indoor-climate pollutants and bases for their risk assessment. Scand J Work Environ Health. 1995;21:165–178. doi: 10.5271/sjweh.25. [DOI] [PubMed] [Google Scholar]
- Nielsen GD, Bakbo JC, Holst E. Sensory irritation and pulmonary irritation by airborne allyl acetate, allyl alcohol, and allyl ether compared to acrolein. Acta Pharmacol Toxicol (Copenh) 1984;54(4):292–298. doi: 10.1111/j.1600-0773.1984.tb01933.x. [DOI] [PubMed] [Google Scholar]
- Nielsen GD, Hougaard KS, Larsen ST, Hammer M, Wolkoff P, Clausen PA, et al. Acute airway effects of formaldehyde and ozone in BALB/c mice. Hum Exp Toxicol. 1999;18:400–409. doi: 10.1191/096032799678840246. [DOI] [PubMed] [Google Scholar]
- Nielsen GD, Yamagiwa M. Structure-activity relationships of airway irritating aliphatic amines. Receptor activation mechanisms and predicted industrial exposure limits. Chem Biol Interact. 1989;71(2–3):223–244. doi: 10.1016/0009-2797(89)90037-9. [DOI] [PubMed] [Google Scholar]
- Pazdrak K, Gorski P, Krakowiak A, Ruta U. Changes in nasal lavage fluid due to formaldehyde inhalation. Int Arch Occup Environ Health. 1993;64(7):515–519. doi: 10.1007/BF00381101. [DOI] [PubMed] [Google Scholar]
- Plotnikova MM. Basic Investigations for the Determination of the Limit of Allowable Acrolein Concentration in Atmospheric Air. Washington, DC: U.S. Department of Commerce; 1960. [Google Scholar]
- Pozzani UC, Carpenter CP. Methyl Isocyanate, Special Report 26-23. Pittsburgh, PA: Mellon Institute; 1963. [Google Scholar]
- Rotman HH, Fliegelman MJ, Moore T, Smith RG, Anglen DM, Kowalski CJ, et al. Effects of low concentrations of chlorine on pulmonary function in humans. J Appl Physiol. 1983;54(4):1120–1124. doi: 10.1152/jappl.1983.54.4.1120. [DOI] [PubMed] [Google Scholar]
- Sangha GK, Alarie Y. Sensory irritation by toluene diisocyanate in single and repeated exposures. Toxicol Appl Pharmacol. 1979;50(3):533–547. doi: 10.1016/0041-008x(79)90408-3. [DOI] [PubMed] [Google Scholar]
- Sauder LR, Chatham MD, Green DJ, Kulle TJ. Acute pulmonary response to formaldehyde exposure in healthy nonsmokers. J Occup Med. 1986;28(6):420–424. doi: 10.1097/00043764-198606000-00008. [DOI] [PubMed] [Google Scholar]
- Sauder LR, Green DJ, Chatham MD, Kulle TJ. Acute pulmonary response of asthmatics to 3.0 ppm formaldehyde. Toxicol Ind Health. 1987;3(4):569–578. doi: 10.1177/074823378700300408. [DOI] [PubMed] [Google Scholar]
- Schachter EN, Witek TJ, Jr, Brody DJ, Tosun T, Beck GJ, Leaderer BP. A study of respiratory effects from exposure to 2.0 ppm formaldehyde in occupationally exposed workers. Environ Res. 1987;44(2):188–205. doi: 10.1016/s0013-9351(87)80227-x. [DOI] [PubMed] [Google Scholar]
- Schachter EN, Witek TJ, Jr, Tosun T, Leaderer BP, Beck GJ. A study of respiratory effects from exposure to 2 ppm formaldehyde in healthy subjects. Arch Environ Health. 1986;41(4):229–239. doi: 10.1080/00039896.1986.9938338. [DOI] [PubMed] [Google Scholar]
- Schaper M. Development of a database for sensory irritants and its use in establishing occupational exposure limits. Am Ind Hyg Assoc J. 1993;54(9):488–544. doi: 10.1080/15298669391355017. [DOI] [PubMed] [Google Scholar]
- Seeber A, Kiesswetter E, Vangala RR, Blaszkewicz M, Golka K. Combined exposure to organic solvents: an experimental approach using acetone and ethyl acetate. Appl Psychol Int Rev. 1992;41(3):281–292. [Google Scholar]
- Shusterman D, Matovinovic E, Salmon AG. Does Haber’s Law apply to human sensory irritation? Inhal Toxicol. 2006;18:457–471. doi: 10.1080/08958370600602322. [DOI] [PubMed] [Google Scholar]
- Silverman L, Schulte HF, First MW. Further studies on sensory response to certain industrial solvent vapors. J Ind Hyg Toxic. 1946;28(6):262–266. [PubMed] [Google Scholar]
- Smyth HF, Jr, Kinkead ER, Pozzani UCS. Methyl isocyanate: acute inhalation toxicity humans responses to low concentrations, guinea pig sensitization and cross sensitization to other isocyanates. In: Browning JB, editor. Compilation of Toxicology on Methyl Isocyanate. Pittsburgh, PA: Mellon Institute; 1970. pp. 21–28. [Google Scholar]
- Steinhagen WH, Barrow CS. Sensory irritation structure-activity study of inhaled aldehydes in B6C3F1 and Swiss-Webster mice. Toxicol Appl Pharmacol. 1984;72(3):495–503. doi: 10.1016/0041-008x(84)90126-1. [DOI] [PubMed] [Google Scholar]
- Stephens ER, Darley EF, Taylor OC, Scott WE. Photochemical reaction products in air pollution. Int J Air and Water Poll. 1961;4(1/2):79–100. [Google Scholar]
- Stewart RD, Dodd HC, Baretta ED, Schaffer AW. Human exposure to styrene vapor. Arch Environ Health. 1968;16(5):656–662. doi: 10.1080/00039896.1968.10665124. [DOI] [PubMed] [Google Scholar]
- Stewart RD, Hake CL, Wu A, Graff SA, Forster HV, Keeler WH, et al. Acetone: Development of a Biologic Standard for the Industrial Worker by Breath Analysis. NTIS PB82172917. Milwaukee, WI: Medical College of Wisconsin, Milwaukee Department of Environmental Medicine, U.S. Department of Commerce; 1975. [Google Scholar]
- Stone LC, Lawhorne GT, McKinney JC, McCracken MS. Upper respiratory tract sensory responses to volatile chemicals. Toxicologist. 1981;1:134. [Google Scholar]
- Sundblad BM, Larsson BM, Acevedo F, Ernstgard L, Johanson G, Larsson K, et al. Acute respiratory effects of exposure to ammonia on healthy persons. Scand J Work Environ Health. 2004;30(4):313–321. doi: 10.5271/sjweh.800. [DOI] [PubMed] [Google Scholar]
- Tomas T, Oliskiewicz W, Czerczak S, Sokal J. Decrease in the respiration rate in mice as an indicator of the irritating effects of chemical substances on the upper respiratory tract [in Polish] Med Pr. 1985;36(5):295–302. [PubMed] [Google Scholar]
- U.S. EPA. Benchmark Dose Software (BMDS): Benchmark Dose Methodology. Washington, DC: U.S. Environmental Protection Agency; 2007. [[accessed 10 October 2007]]. Available: http://www.epa.gov/ncea/bmds/bmds_training/methodology/intro.htm. [Google Scholar]
- Verberk MM. Effects of ammonia in volunteers. Int Arch Occup Environ Health. 1977;39(2):73–81. doi: 10.1007/BF00380887. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan R, Schaper M, Thompson R, Stock MF, Boylstein LA, Luo JE, et al. Computer assisted recognition and quantitation of the effects of airborne chemicals acting at differing areas of the respiratory tract. Arch Toxicol. 1994;68:490–499. doi: 10.1007/s002040050101. [DOI] [PubMed] [Google Scholar]
- Von Nieding G, Wagner HM. Experimental studies on the short-term effect of air pollutants on man: two hour exposure to NO2, O3 and SO2 alone and in combination. In: Kasuga S, Suzuki N, Yamada T, Kimura G, Inagaki K, Onoe K, editors. Proceedings of the Fourth International Clean Air Conference. Tokyo, Japan: Japanese Union of Air Pollution Prevention Associations; 1977. pp. 5–8. [Google Scholar]
- Weber-Tschopp A, Fischer T, Grandjean E. Irritating effects of formaldehyde on man [in German] Int Arch Occup Environ Health. 1977;39(4):207–218. doi: 10.1007/BF00409367. [DOI] [PubMed] [Google Scholar]
- Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, Oyen F. Toxicological studies of certain alkylated benzenes and benzene; experiments on laboratory animals. AMA Arch Ind Health. 1956;14(4):387–398. [PubMed] [Google Scholar]