Abstract
The oxidation hypothesis of atherogenesis has been the focus of much research over the past two decades. However, randomized placebo-controlled trials evaluating the efficacy of vitamin E to prevent cardiovascular events in aggregate have failed to show a beneficial effect. Implicit in these trials is that the dose of vitamin E tested effectively suppressed oxidative stress status but this was never determined. We defined the dose-dependent effects of vitamin E (RRR-α-tocopherol) to suppress plasma concentrations of F2-isoprostanes, a biomarker of free radical mediated lipid peroxidation, in participants with polygenic hypercholesterolemia and enhanced oxidative stress, a population at risk for cardiovascular events. A time-course study was first performed in participants supplemented with 3200 I.U./day of vitamin E for 20 weeks. A dose-ranging study was then performed in participants supplemented with 0, 100, 200, 400, 800, 1600, or 3200 I.U./day of vitamin E for 16 weeks. In the time-course study, maximum suppression of plasma F2-Isoprostane concentrations did not occur until 16 weeks of supplementation. In the dose-ranging study there was a linear trend between the dosage of vitamin E and percent reduction in plasma F2-isoprostane concentrations which reached significance at doses of 1600 I.U (35% ± 2% p<0.035) and 3200 I.U (49% ± 10% p<0.005). This study provides information on the dosage of vitamin E that decreases systemic oxidant stress in vivo in humans and informs the planning and evaluation of clinical studies that assess the efficacy of vitamin E to mitigate disease.
Keywords: Oxidative stress, vitamin E, free radicals, isoprostane, cardiovascular disease, hypercholesterolemia
INTRODUCTION
Previous epidemiologic studies suggested an inverse relationship between dietary intake and plasma concentrations of vitamin E and risk of cardiovascular disease (1, 2). Moreover, vitamin E administration reduces atherogenesis in some but not all animal models of atherosclerosis (3).
Underpinning the interest in the potential ability of antioxidants to prevent cardiovascular disease is the oxidation hypothesis of atherogenesis (4). Normal low density lipoprotein (LDL) is taken up in limited amounts by macrophages. However, oxidatively modified LDL is readily taken up by macrophages via scavenger receptor pathways, leading to the formation of foam cells. In this regard, it has been shown that reactive aldehydes that are formed as products of free radical mediated lipid peroxidation can modify apolipoprotein B to a form that is more negatively charged and recognized by scavenger receptors (5). Atherosclerotic plaques in both humans and animals also contain increased levels of oxidized lipids (6).
Because of the association of increased free radical formation in atherogenesis, a number of large randomized placebo-controlled clinical trials have previously explored the efficacy of supplementation with the antioxidant vitamin E (α-tocopherol) to prevent cardiovascular events (7–15). In aggregate, these studies have failed to show a preventive effect of vitamin E on cardiovascular events even at dosages up to 900 IU/d (16). Implicit in these studies is that the dose of vitamin E tested significantly reduced the level of oxidative stress in the study participants. Even though the importance of determining this has been emphasized (17), this was only addressed in a small cohort in one of the studies (18). In this study, no significant difference was found in the urinary excretion of the F2-isoprostane, 15-F2t-isoprostane, a biomarker of free radical mediated lipid peroxidation, in participants who were supplemented with vitamin E compared to those who were not supplemented with vitamin E. In the absence of knowledge regarding the relationship between dose of vitamin E and suppression of oxidative stress, it is difficult, if not impossible, to draw any firm conclusions from the results of these studies as to the role of oxidative stress in cardiovascular disease.
Therefore, we undertook to define the clinical pharmacology of vitamin E as an antioxidant in individuals with hypercholesterolemia. We and others have shown that polygenic hypercholesterolemia in humans is associated with enhanced oxidative stress (19, 20). While the mechanistic basis for this remains unclear, we believed that individuals with hypercholesterolemia would be an ideal study group in which to define the clinical pharmacology of vitamin E because of the association of hypercholesterolemia and increased risk for cardiovascular disease.
Two studies were undertaken. We first performed a time-course study with the highest dose of natural vitamin E (RRR-α-tocopherol) that we tested (3200 I.U./day) to determine the length of time required to reach maximum suppression of oxidative stress. This was an important study to undertake because it has been shown that whereas plasma concentrations of vitamin E increase and plateau fairly rapidly during daily supplementation with vitamin E, tissue concentrations of vitamin E reach steady-state concentrations very slowly over a period of several weeks (21). The results from the time-course study then informed our second study, a dose-ranging study. Oxidative stress status was monitored by measurements of plasma concentrations of F2-isoprostanes. F2-isoprostanes are prostaglandin F2-like compounds that are formed non-enzymatically by free radical mediated peroxidation of arachidonic acid (22). Measurement of F2-isoprostanes by mass spectrometry is now widely recognized as one of the most accurate and reliable approaches to assess oxidative stress status in vivo (23, 24).
METHODS
Participants
These studies were approved by the Vanderbilt University Institutional Review Board and all participants gave informed consent. Participants with polygenic hypercholesterolemia were studied. Participants that had a total serum cholesterol level higher than 200 mg/dl at the initial screening evaluation were selected for entry into the study. Participants with any known disease except for minor non-systemic ailments were excluded. Participants who smoked or were taking multivitamins or other known antioxidants were also excluded. Participants were instructed on a diet that delivered a fairly constant intake of vitamins from food. Participants taking lipid-lowering drugs were also excluded because some of these drugs may have antioxidant properties (20). Participants with serum cholesterol levels above 200 mg/dl were then further screened for the presence of oxidative stress by measuring the concentration of F2-isoprostanes in their plasma and were excluded from the study if their plasma concentration of F2-isoprostanes was not higher than 2 S.D. above the normal mean (35 ± 12 pg/ml; mean ± 2 S.D.).
Time-Course Study
3200 I.U. of natural vitamin E (RRR-d-α-tocopherol acetate) (E-Gems, J.R. Carlson Laboratories, Arlington Heights, Ill) was administered daily to 8 participants (34 ± 4 years of age) for 20 weeks. One subject was male and seven were females. Plasma concentrations of F2-Isoprostanes were measured at bi-weekly intervals. Total serum cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and vitamin E levels were measured prior to beginning the study and again at the end of the study.
Dose-Ranging Study
This was a double blind randomized placebo-controlled study. Thirty-five participants were randomized to receive either placebo, 100, 200, 400, 800, 1600, or 3200 I.U. of vitamin E daily for 16 weeks. Twelve of the participants were males and 23 were females and the mean age of the participants was 42 ± 2 years. Plasma concentrations of F2-isoprostanes and total serum cholesterol were measured at week 0 and at the end of week 16. Participants were seen monthly during this study to review any questions or issues about the study and to determine compliance by pill counts.
For both the time-course and dose-ranging studies, the majority of participants were women. Female gender is associated with higher F2-isoprostane levels but the extent of reduction of isoprostanes by vitamin E in both studies was unaffected by gender.
Analytical Methods
Alpha-tocopherol was quantitated by the method of Craft as modified by Gross et al. (25). F2-isoprostanes were measured by gas chromatography mass spectrometric assay (26). The precision of this assay is ± 6% and the accuracy is 96%. Plasma concentrations of cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were measured as described (27, 28).
Statistical Methods
Analyses of trial results focused on estimating the changes in levels of F2-isoprostanes and vitamin E in the time-course study and the dose-ranging study. The trend of the time-course study over the 20-week study period was analyzed using the restricted/residual maximum likelihood (REML) based mixed effect model to adjust the intra-correlation effect for subjects who had multiple comparisons. The Akaike’s Information Criterion (AIC) was applied for the model selection. The student t-test was applied for the dose-ranging study initially. The multiple testing problem of the dose-ranging study was adjusted by using the permutation t-test and the multiplicity-adjusted p-value was reported. All tests of significance were two-sided and differences were considered statistically significant when p-value <0.05. All data are expressed as means (± SEM). S-Plus 7.0 and SAS version 9.1 was used for all analyses.
RESULTS
Time-Course Study
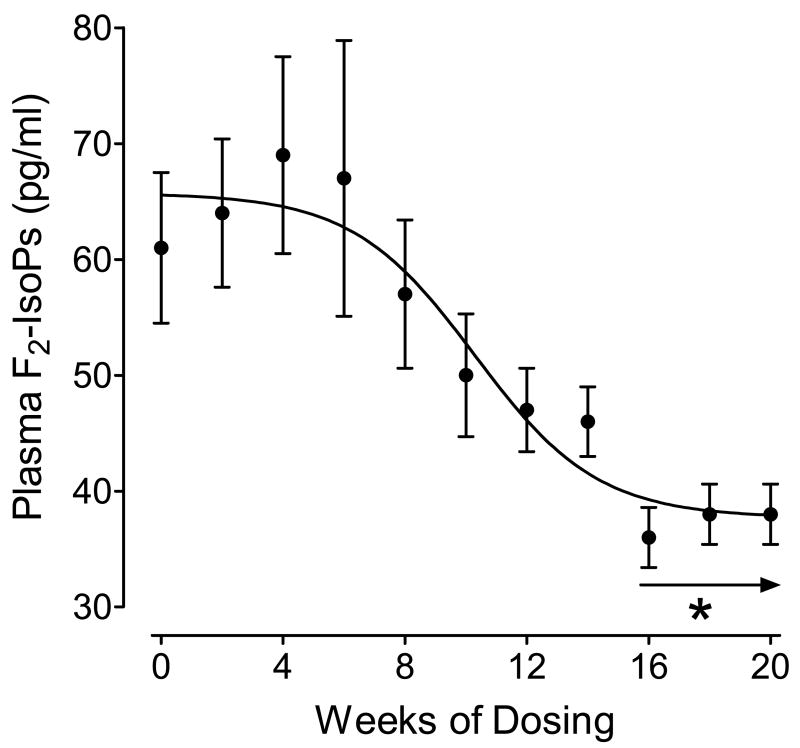
The time-course of the effect of daily administration of 3200 I.U./day of vitamin E on reduction of plasma concentrations of F2-isoprostanes is shown in Figure 1. Repeated measures of trend analysis showed a significant overall time-effect on suppression of plasma concentrations of F2-isoprostanes by vitamin E (p<0.001). A significant reduction from mean plasma concentrations of F2-isoprostanes at week 0 (61.7 ± 6.5 pg/ml) did not occur until 16 weeks of vitamin E supplementation (36.3 ± 2.6 pg/ml) (p<0.005) and levels remained suppressed through weeks 18 and 20 (p<0.005). Plasma concentrations of vitamin E were significantly increased at week 20 compared to week 0 but levels of total serum cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were not significantly different at week 0 and week 20 (Table 1).
Figure 1.
Time course of reduction in plasma concentrations of F2-isoprostanes (F2-IsoPs) in participants supplemented with 3200 I.U./day of vitamin E. *−p<0.005 compared to Time 0.
TABLE 1.
Levels of Plasma Lipids and Vitamin E at the Beginning and End of the Time-Course Study
| Study Start | Study End | ||
|---|---|---|---|
| Cholesterol | 256 ± 23 mg/dl | 242 ± 22 mg/dl | *N.S. |
| LDL Cholesterol | 158 ± 23 | 141 ± 26 | N.S. |
| HDL Cholesterol | 58 ± 8 | 58 ± 6 | N.S. |
| Triglycerides | 193 ± 78 | 211 ± 81 | N.S. |
| Vitamin E | 1.48 ± 0.24 | 4.01 ± 0.81 | <0.05 |
N.S. = not significant
Dose-Ranging Study
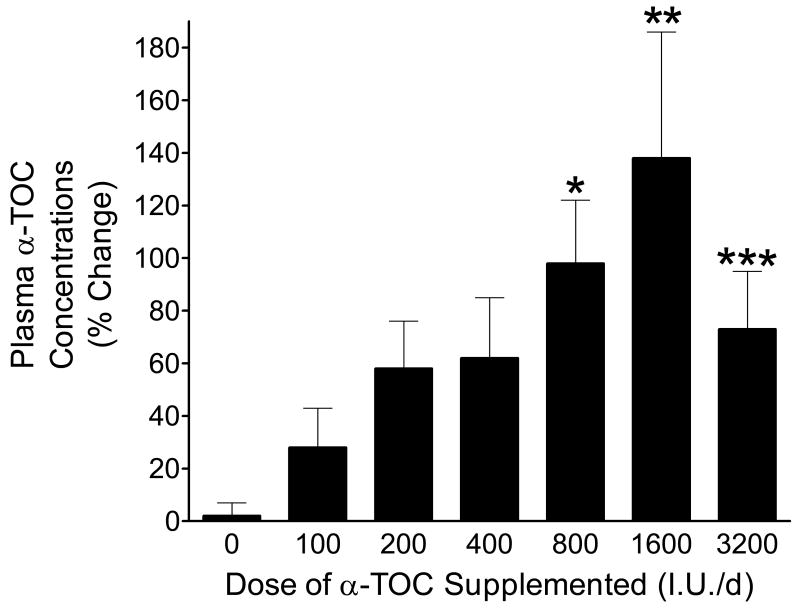
Plasma concentrations of vitamin E at week 16 in participants taking varying doses of vitamin E and placebo are shown in Figure 2. Multiple comparisons showed that the percent changes in plasma vitamin E concentrations at doses of 800, 1600, and 3200 mgs were statistically higher than in the placebo group (p<0.004, p<0.001, p<0.01, respectively). There was also a linear trend between plasma concentration and dosage (p=0.009). Although the mean level of vitamin E in participants supplemented with 3200 I.U/day is less than the level measured in participants taking 1600 I.U/day, it is recognized that plasma concentrations of vitamin E do not correlate with tissue levels and that plasma concentrations are highly variable, especially during supplementation with high doses of vitamin E (29). In addition, plasma concentrations of vitamin E are highly dependent on plasma lipid levels and the variable levels of alpha-tocopherol observed in the present studies may have, in part, been a consequence of variations in plasma lipids. On the other hand, elevated plasma concentrations of vitamin E primarily indicate that the participants were taking their vitamin E. Compliance with taking the prescribed vitamin E was confirmed by pill counts performed during monthly visits to the Vanderbilt Clinical Trials Center. As with the time-course study, vitamin E, at all doses examined, had no effect on serum cholesterol or triglyceride levels in participants.
Figure 2.
Plasma concentrations of vitamin E measured after 16 weeks of supplementation with varying doses of vitamin E or placebo. *−p<0.004 compared to placebo; **−p<0.001 compared to placebo; ***−p<0.01 compared to placebo.
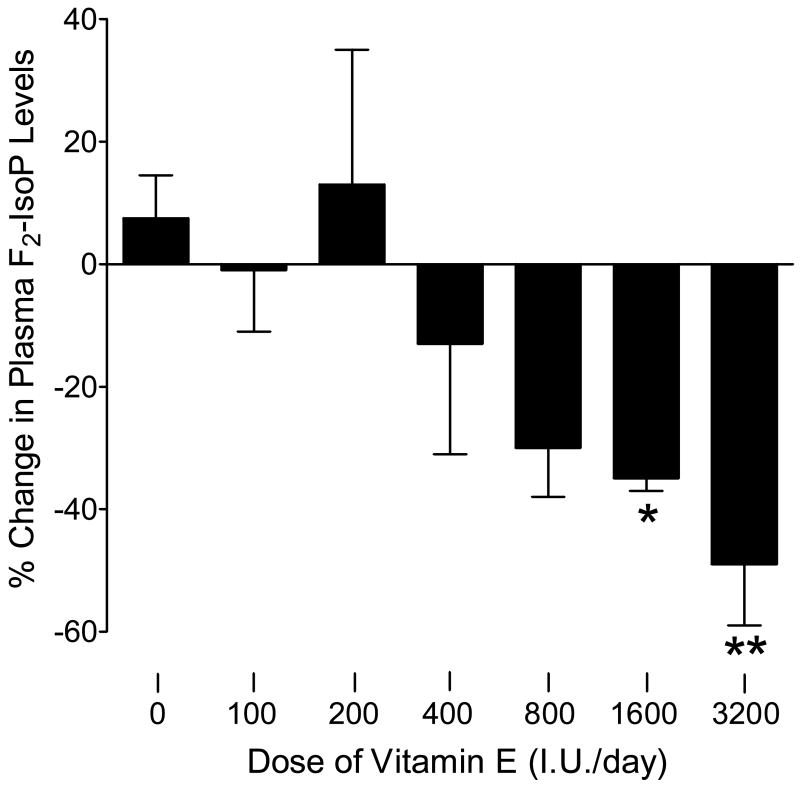
The effect of supplementation with varying doses of vitamin E for 16 weeks on plasma F2-isoprostane concentrations is shown in Figure 3. There was a significant linear trend between dosage of vitamin E and percent change in plasma concentrations of F2-isoprostanes (p<001). There was an obvious suppression by vitamin E at 800 I.U. (30 ± 8%; p=0.094) which reached significance at 1600 I.U (35 ± 2%; p<0.03) and 3200 I.U (49 ± 10%; p<0.005).
Figure 3.
Relationship between the daily dosages of vitamin E administered for 16 weeks and suppression of plasma concentrations of F2-isoprostanes (F2-IsoPs). *−p<0.03 compared to placebo; **−p<0.005 compared to placebo.
DISCUSSION
This study has defined the clinical pharmacology of vitamin E in humans with increased oxidative stress associated with polygenic hypercholesterolemia. The goal of these experiments was to obtain information regarding the relationship between dose of vitamin E and suppression of oxidative stress in humans and the duration of treatment required to achieve this effect. This information is essential for the interpretation of clinical studies that have been performed exploring the ability of vitamin E, and other putative antioxidants, to mitigate disease processes in humans. In addition, this information could inform future studies evaluating the effects of vitamin E and other antioxidants in disease states associated with oxidative stress about appropriate doses to evaluate treatment required for a significant effect.
With this data in hand, we may now be able to more rationally interpret the findings from the large placebo-controlled randomized primary and secondary clinical prevention trials that evaluated the ability of vitamin E supplementation to reduce the occurrence of cardiovascular events, which included fatal or non-fatal myocardial infarction and stroke (7–15). A meta-analysis of these trials found a lack of significant benefit (16). In addition, other randomized placebo-controlled trials examined the ability of vitamin E at doses of (a) 800 I.U., type unspecified, in combination with 500 mg of vitamin C (WAVE trial), (b) 136 I.U all-rac-α-tocopherol in combination with 250 mg of vitamin C (ASAP trial), and (c) 400 I.U. RRR-α-tocopherol (VEAPS trial) to suppress atherosclerotic progression rather than cardiovascular events (30–32). The VEAPS trial which evaluated the effect of 400 I.U of all-rac-α-tocopherol and the WAVE trial which evaluated the effect of 800 I.U of vitamin E (type not specified) in combination with 500 mg of vitamin C found no effect on the atherosclerotic progression. The ASAP trial which evaluated 136 I.U. of all-rac-α-tocopherol in combination with 250 mg of vitamin C did observe a significant 25% reduction in atherosclerotic progression. One randomized placebo-controlled trial (SPACE) examined the effect of supplementation of 800 I.U of RRR-α-tocopherol alone in patients on hemodialysis and found a significant 54% reduction in cardiovascular events (33). However, this may be a unique group of patients because they have low intake of vitamin E and abnormalities in levels and metabolism of vitamin E such that the potency of vitamin E administration would be expected to be significantly enhanced to a greater degree compared to patients who are not on hemodialysis (34). An additional randomized placebo-controlled trial (SU.VI.MAX study) in which participants were supplemented with 45 I.U of vitamin E in combination with other antioxidants, vitamin C, beta carotene, zinc, and selenium, also failed to find a significant reduction in cardiovascular events (35). The fact that in aggregate these trials have not found significant benefit has called into question the validity of the oxidation hypothesis of atherosclerosis (17). We found a significant linear trend between the dosage of vitamin E and the percent reduction in plasma F2-isoprostane concentrations. However, a significant suppression in plasma F2-isoprostane concentrations was only observed at doses of 1600 and 3200 I.U./day. It should also be pointed out that the form of vitamin E administered in our study was RRR-α-tocopherol, which has about twice the bioavailability compared to synthetic all-rac-α-tocopherol (36). In that regard and in light of the fact that there was an obvious reduction, albeit not quite statistically significant, in plasma concentrations of F2-isoprostanes in participants in our study supplemented with 800 I.U./day of RRR--α-tocopherol, it is of interest that the one clinical trial that evaluated 800 I.U./day of RRR--α-tocopherol (CHAOS) did observe a 47% reduction in cardiovascular events.
It should be noted that implicit in the design of our study is the assumption that vitamin E is an antioxidant in humans. In vitro literature suggest that it may be a pro-oxidant at certain concentrations but our data, and that of others, suggests that over a very wide dose range that this is not the case in vivo (37,38).
A similar study to ours has been performed by Meagher and colleagues but with important differences in study design and findings (39). In that study, normal participants were supplemented with 200, 400, 800, 1200, or 2000 I.U. of vitamin E (RRR-α-tocopherol)/day for 8 weeks. They found no effect on the suppression of the formation of F2-isoprostanes or urinary excretion of 4-hydroxynonenal, another product of lipid peroxidation. The authors speculated that the failure to find suppression in levels of F2-isoprostanes may likely attributed to the fact that the level of oxidative stress in the individuals in this study was not elevated. However, other studies in which normal healthy individuals were supplemented with soy isoflavones or aged garlic extract, both of which have antioxidant properties, showed significant reductions in levels of F2-isoprostanes (40, 41). Based on our findings in our time-course study, a more likely explanation for their failure to find a suppression of the formation of F2-isoprostanes is because the duration of their study, 8 weeks, was too short to observe the full suppressive effect of vitamin E on levels of F2-isoprostanes. In support of the contention that long term, high dose vitamin E decreases oxidant stress, a recent study reported an 11% reduction (p=0.03) in plasma isoprostanes in obese individuals supplemented with 800 IU natural alpha-tocopherol for six months (42).
There was a significant linear trend between dosage of vitamin E and percent change in plasma concentrations of F2-isoprostanes but the magnitude of the reduction was statistically significant only at doses of 1600 I.U./day and 3200 I.U./day. There was no clinically apparent toxicity in this study, even at the high dose of 3200 I.U. Two studies have suggested, however, that high doses of vitamin E (≥ 400 I.U/day) may increase the risk of all cause mortality (43) and heart failure (44) and a recent meta-analysis of antioxidant supplements for the prevention of several diseases including atherosclerosis reported an increased mortality with vitamin E and other agents (45). However, a comprehensive review of all published safety observations for vitamin E supplementation in clinical trials concluded that vitamin E supplements appear safe for most adults in amounts ≤1600 I.U. of RRR-α-tocopherol (46). Another review of a number of double-blind controlled studies revealed no important adverse effects associated with vitamin E supplementation at intakes ranging up to 3200 I.U./day (47). Nonetheless, based on these conflicting reports, long term treatment with high dose vitamin E cannot be justified at this time. It should also be pointed out that the mean maximum suppression of plasma concentrations of F2-isoprostanes seen in participants who were supplemented with the largest dose of vitamin E, 3200 I.U./day, was 49%, which is not a profound reduction. This suggests that the antioxidant potency of vitamin E in vivo in humans is not great.
The results of this study should provide a framework for future studies assessing the ability of therapeutic agents to suppress oxidant stress in humans. Specifically, we have now what is generally recognized to be a reliable approach to assess oxidative stress status in humans, i.e. measurement of F2-isoprostanes. Therefore, prior to initiating large clinical trials of agents that alter oxidant stress, investigators should establish that the dose(s) of the compound tested does in fact significantly reduce oxidative stress status in the study participants. Ideally such measurements should also be incorporated in the study design in the participant population studied to monitor efficacy and variability of the pharmacologic intervention to reduce oxidative stress status across a larger cohort of participants. Such data can be invaluable in the interpretation of results obtained when correlated with the magnitude of suppression of oxidative stress in the participants studied.
Acknowledgments
This work was supported by NIH grants GM42056 (MERIT Award to LJR), HL65709 and HL57986 (to SF), HL58427 (to MFL), DK48831, GM15431 and ES13125 (to JM) and DK26657.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 2002;328:1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 3.Upston JM, Kritharides L, Stocker R. The role of vitamin E in atherosclerosis. Prog Lipid Res. 2003;42:405–422. doi: 10.1016/s0163-7827(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 5.Steinbrecher UP. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987;262:3603–3608. [PubMed] [Google Scholar]
- 6.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virtamo J, Rapola JM, Ripatti S, Heinonen OP, Taylor PR, Albanes D, Huttunen JK. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Intern Med. 1998;158:668–675. doi: 10.1001/archinte.158.6.668. [DOI] [PubMed] [Google Scholar]
- 8.Rapola JM, Virtamo J, Ripatti S, Huttunen JK, Albanes D, Taylor PR, Heinonen OP. Randomised trial of alpha-tocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet. 1997;349:1715–1720. doi: 10.1016/S0140-6736(97)01234-8. [DOI] [PubMed] [Google Scholar]
- 9.de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 10.Lonn E, Yusuf S, Dzavik V, Doris C, Yi Q, Smith S, Moore-Cox A, Bosch J, Riley W, Teo K Secure Investigators. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE) Circulation. 2001;103:919–95. doi: 10.1161/01.cir.103.7.919. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–10. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 12.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenias GR HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–13. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 13.Investigators GP. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 14.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 15.Group HPSC. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2003;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 16.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 17.Heinecke JW. Is the emperor wearing clothes? Clinical trials of vitamin E and the LDL oxidation hypothesis. Arterioscler Thromb Vasc Biol. 2001;21:1261–1264. doi: 10.1161/hq0801.095084. [DOI] [PubMed] [Google Scholar]
- 18.Chiabrando C, Avanzini F, Rivalta C, Colombo F, Fanelli R, Palumbo G, Roncaglioni MC. PPP Collaborative Group on the antioxidant effect of vitamin E. Long-term vitamin E supplementation fails to reduce lipid peroxidation in people at cardiovascular risk: analysis of underlying factors. Curr Control Trials Cardiovasc Med. 2002;19:5. doi: 10.1186/1468-6708-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts LJ, 2nd, Morrow JD. Isoprostanes as markers of lipid peroxidation in atherosclerosis. In: Serhan PAWCN, editor. Molecular and Cellular Basis of Inflammation. Totowa: Humana Press; 1999. pp. 141–163. [Google Scholar]
- 20.Davi G, Alessandrini P, Mezzetti A, Minotti G, Bucciarelli T, Costantini F, Cipollone F, Bon GB, Ciabattoni G, Patrono C. In vivo formation of 8-Epi-prostaglandin F2 alpha is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:3230–3235. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa M, Mino M. Effects of elevated d-alpha(RRR)-tocopherol dosage in man. J Nutr Sci Vitaminol (Tokyo) 1989;35:133–142. doi: 10.3177/jnsv.35.133. [DOI] [PubMed] [Google Scholar]
- 22.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–13. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 24.Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, Basu S, FitzGerald GA, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaris J, Roberts LJ, Rokach J, Shigenaga MK, Sun J, Walter PB, Tomer KB, Barrett JC, Mason RP. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl(4) poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Gross MD, Yu X, Hannan P, Prouty C, Jacobs DR. Lipid standardization of serum fat-soluble antioxidant concentrations: the YALTA study. Am J Clin Nutr. 2003;77:458–466. doi: 10.1093/ajcn/77.2.458. [DOI] [PubMed] [Google Scholar]
- 26.Morrow JD, Roberts LJII. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 1998;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 27.Linton; MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 28.Gross MD, Prouty CB, Jacobs DR., Jr Stability of carotenoids and alpha-tocopherol during blood collection and processing procedures. Clin Chem. 1995;41:943–944. [PubMed] [Google Scholar]
- 29.Traber MG, Rader D, Acuff RV, Ramakrishnan R, Brewer HB, Kayden HJ. Vitamin E dose-response studies in humans with use of deuterated RRR-alpha-tocopherol. Am J Clin Nutr. 1998;68:847–853. doi: 10.1093/ajcn/68.4.847. [DOI] [PubMed] [Google Scholar]
- 30.Salonen RM, Nyyssonen K, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Rissanen TH, Tuomainen TP, Valkonen VP, Ristonmaa U, Lakka HM, Vanharanta M, Salonen JT, Poulsen HE. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation. 2003;107:947–953. doi: 10.1161/01.cir.0000050626.25057.51. [DOI] [PubMed] [Google Scholar]
- 31.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, Liu CH, Hwang J, Selzer RH, Azen SP VEAPS Research Group. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 32.Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, Ouyang P, Thompson P, Tardif JC, Higginson L, Bittner V, Steffes M, Gordon DJ, Proschan M, Younes N, Verter JI. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 33.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 34.Galli F, Buoncristiani U, Conte C, Aisa C, Floridi A. Vitamin E in uremia and dialysis patients. Ann N Y Acad Sci. 2004;1031:348–351. doi: 10.1196/annals.1331.041. [DOI] [PubMed] [Google Scholar]
- 35.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briancon S. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 36.Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 37.Witting PK, Upston JM, Stocker R. The molecular action of alpha-tocopherol in lipoprotein lipid oxidation. Pro- and antioxidant activity of vitamin E in complex heterogeneous lipid emulsions. Subcell Biochem. 1998;30:345–390. [PubMed] [Google Scholar]
- 38.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meagher EA, Barry OP, Lawson JA, Rokach J, FitzGerald GA. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA. 2001;285:1178–1182. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- 40.Dillon SA, Lowe GM, Billington D, Rahman K. Dietary supplementation with aged garlic extract reduces plasma and urine concentrations of 8-iso-prostaglandin F(2 alpha) in smoking and nonsmoking men and women. J Nutr. 2002;132:168–171. doi: 10.1093/jn/132.2.168. [DOI] [PubMed] [Google Scholar]
- 41.Wiseman H, O’Reilly JD, Adlercreutz H, Mallet AI, Bowey EA, Rowland IR, Sanders TA. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 2000;72:395–400. doi: 10.1093/ajcn/72.2.395. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland WH, Manning PJ, Walker RJ, de Jong SA, Ryalls AR, Berry EA. Vitamin E supplementation and plasma 8-isoprostane and adiponectin in overweigth subjects. Obesity (Silver Spring) 2007;15:386–391. doi: 10.1038/oby.2007.546. [DOI] [PubMed] [Google Scholar]
- 43.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 44.Investigators THaH-TT. Effects of long-term vitamin E supplementation on cardiovascular events and cancer. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 45.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 46.Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, Jialal I, Jonston CS, Kelly FJ, Kraimer K, Packer L, Parthasarathy S, Sies H, Traber MG. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–745. doi: 10.1093/ajcn/81.4.736. [DOI] [PubMed] [Google Scholar]
- 47.Kappus H, Diplock AT. Tolerance and safety of vitamin E: a toxicological position report. Free Radic Biol Med. 1992;13:55–74. doi: 10.1016/0891-5849(92)90166-e. [DOI] [PubMed] [Google Scholar]