Migration of neuronal precursor cells from the external germinal layer (EGL) to the internal granular layer (IGL) is a crucial process in the development of the mammalian cerebellar cortex. These cells make up the only precursor population known to migrate away from the surface of the brain. We studied the role of the chemokine stromal-derived factor 1 (SDF-1) in the cerebellar tissue of rats and knockout mice and found (i) that it functions as an attractive guidance cue for neuronal migration and (ii) that its secretion from non-neuronal meningeal tissue is important for controlling the migration of embryonic EGL cells.
SDF-1 was first noted for its role in leukocyte chemotaxis1,2. Notably, mice lacking SDF-1, and its receptor CXCR4, have been found to have a similar cerebellar phenotype: cerebellar granule cells appear prematurely in the internal layers of the embryo3–5. This suggests that SDF-1 is either directly or indirectly involved in positioning the EGL cells3–5. The precise role of SDF-1 in EGL migration is not yet known6,7—the effects of SDF-1 on embryonic EGL cells have never been tested. The migration of dissociated cells from the postnatal IGL in a Transwell assay8 suggests that both SDF-1 and brain-derived neurotrophic factor (BDNF) are attractants for the IGL cells and that Eph-Ephrin signaling inhibits the IGL response to SDF-1. Postnatal IGL cells are derived from EGL cells that have migrated internally and differentiated, and are no longer identical to embryonic or postnatal EGL cells. Because the phenotype of SDF-1 knockout mice is determined during embryogenesis, it is important to investigate the role of SDF-1 in guiding the migration of embryonic EGL cells.
We first used immunohistochemistry to examine the distribution of the SDF-1 protein in the rat cerebellum (Fig. 1). SDF-1 was present in both the embryonic and the postnatal meninges (Fig. 1a and b). The staining with antibody against SDF-1 was done by adding exogenous SDF-1 protein (data not shown). The presence of SDF-1 in the meninges is consistent with a role for SDF-1 in guiding the migration of EGL cells. CXCR4 is expressed in the neuroepithelium, rhombic lip and EGL of the embryonic and postnatal cerebellum3,8,9.
Fig. 1.

Expression of the SDF-1 protein in the meninges. (a) Anti-SDF-1 staining of the cerebellum at embryonic day 15 (E15) shown in red and nuclear staining with Hoechst 33258 dye shown in blue. (b) Anti-SDF-1 and nuclear staining of the cerebellum at birth (P0). Scale bar, 100 μm. M, meninges. EGL, external germinal layer. Protocols for animal experiments were approved by the institutional animal studies reviewing board of Washington University Medical School.
The phenotype of SDF-1 knockout mice may indicate an indirect role for SDF-1 in regulating the environment through which the neurons can migrate3,4. In the nervous system, a single molecule can be either an attractant or repellant, depending on the cell types and intracellular environments of the responding neurons10. SDF-1 serves as either an attractant or a repellant for T lymphocytes11. To test for a potential role of SDF-1 in EGL migration, we co-cultured embryonic rat EGL explants with aggregates of control human embryonic kidney (HEK) cells or HEK cells expressing SDF-1. Control HEK cells did not attract or repel embryonic EGL cells (Fig. 2a), but HEK cells expressing SDF-1 did (Fig. 2b). HEK cells expressing the chemokine RANTES could neither attract nor repel EGL cells (Fig. 2g), indicating that embryonic EGL cells specifically responded to SDF-1 as a chemoattractant. To rule out the possibility that there were indirect effects of SDF-1 expression in HEK cells, we tested the activity of purified proteins by embedding the protein of interest in a collagen block. Embryonic EGL cells were attracted by collagen blocks embedded with the SDF-1 protein (Fig. 2i), but not toward control collagen blocks (Fig. 2h). These results show that SDF-1 protein can directly attract embryonic EGL cells. By contrast, postnatal EGL cells were neither attracted nor repelled by SDF-1 (Fig. 2l).
Fig. 2.
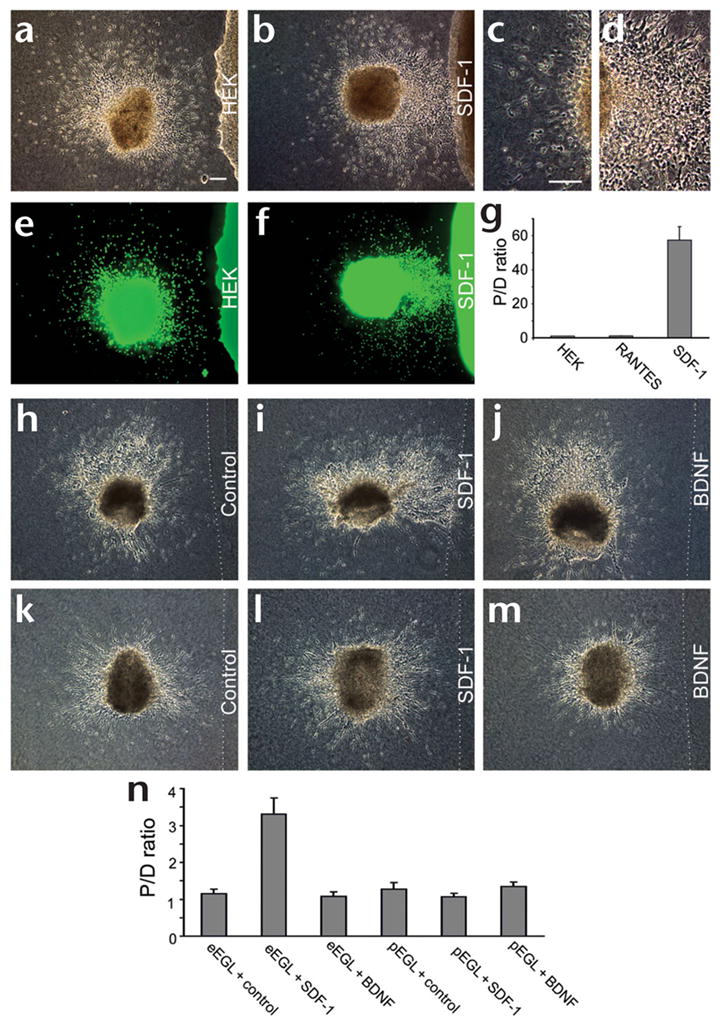
Responses of EGL cells to SDF-1. (a) Co-culture of an E17 EGL explant with control HEK cells. Scale bar, 100 μm. (b) Co-culture of an E17 EGL explant with HEK cells expressing SDF-1. (c) A higher-magnification view of the distal side of the explant shown in (b). (d) A higher-magnification view of the proximal side of the explant shown in (b). (e) Nuclear staining of the explants shown in (a). Green, staining with Hoechst 33258 dye. (f) Nuclear staining of the explants shown in (b). (g) Statistical analysis of EGL responses. The P/D ratio for control HEK cells differed significantly from that for SDF-1 cells (P < 0.0001, Mann-Whitney U-test), but not from that for RANTES cells (P > 0.5). (h–j) Co-culture of embryonic EGL explants with a control block (h), a collagen block containing recombinant SDF-1 (i) or a collagen block containing BDNF (j). SDF-1 (0.04 μM) was sufficient to attract embryonic EGL cells. BDNF did not attract EGL cells even at a concentration of 1.5 μM. (k–m) Co-culture of postnatal explants with a control block (k), a collagen block containing recombinant SDF-1 (l) or a collagen block containing BDNF (m). Postnatal EGL cells were not attracted or repelled by SDF-1 at concentrations as high as 1.0 μM. (n) Statistical analysis of EGL responses toward protein-containing collagen blocks. The P/D ratio for eEGL + SDF-1 differed significantly from that for eEGL + control (P < 0.01, Mann-Whitney U-test), whereas other comparisons did not show a significant difference from the controls (P = 1.000 for eEGL + BDNF, P = 0.107 for pEGL + SDF-1, P = 0.572 for pEGL + BDNF). The latter two were compared to pEGL + control. See Supplementary Methods online for details of these experiments.
Based on the phenotype of SDF-1 knockout mice, it has been previously suggested that SDF-1 acts by inhibiting neuronal differentiation4. A second hypothesis is that SDF-1 increases cell adhesion and thus immobilizes EGL cells3. Both of these suggestions predict that SDF-1 would inhibit EGL migration, contrary to our finding of EGL migration toward SDF-1. Because our data indicate that SDF-1 is an attractant made by the meninges, the mouse knockout phenotype can now be interpreted as evidence for an essential in-vivo role of SDF-1 in attracting EGL cells towards the meninges, and thereby anchoring them.
A recent study uses the Transwell assay to examine neuronal migration8. This assay is not ideal for studying neuronal guidance because neurons isolated from postnatal IGL are dissociated and placed in a chamber, separated from SDF-1 and BDNF by a filter. The number of IGL cells that migrate across the filter is used as a measure of chemoattraction, and as both SDF-1 and BDNF increased the number of cells that migrate, it was concluded that SDF-1 and BDNF are both chemoattractants for IGL cells8. Although chemoattraction cannot be ruled out, an alternative interpretation is that there was an increase in cell motility. Our explant co-culture assays, by contrast, measured the ratio of cells in the proximal and distal parts of the explants and are thus better indicators of chemoattraction.
Although it was known that SDF-1 is expressed in the meninges and exogenous SDF-1 attracts embryonic EGL cells, it was not known whether endogenous SDF-1 is the major attractant in the meninges. We used mice lacking the SDF-1 gene to investigate the role of endogenous SDF-1 (ref. 2). Explants of meninges from wild-type mice attracted embryonic EGL cells (Fig. 3a and c). The absence of the SDF-1 gene in the knockout mice eliminated the attractive activity in the meninges (Fig. 3b and c). These results demonstrate that the endogenous SDF-1 is either the only or the predominant attractant in the meninges. Embryonic EGL cells were actually repelled by meninges from mice lacking SDF-1 (Fig. 3c), indicating that there is a repellant in the meninges distinct from SDF-1 and that endogenous SDF-1 predominates over the endogenous repellant in the embryonic meninges.
Fig. 3.

Analysis of explants from mice lacking the SDF-1 gene. (a) Nuclear staining of a co-culture of an embryonic EGL explant with the meninges from a wild-type mouse (SDF +/+) at embryonic day (E) 14.5. Scale bar, 100 μm. (b) Nuclear staining of a co-culture of an embryonic EGL explant with the meninges from an SDF null mouse (SDF−/−) at E14.5. Genotyping of SDF-1 knockout mice was done as previously described3. (c) Statistical analysis (34 explants tested for SDF+/+ and 41 for SDF−/−). The difference between SDF+/+ and SDF −/− was statistically significant (P < 0.0001, Mann-Whitney U-test).
In summary, SDF-1 functioned as a chemoattractant secreted from the meninges. It was present in both embryonic and postnatal meninges; it attracted embryonic but not postnatal EGL cells; and it did not act as a repellant for EGL cells. Crucially, the absence of a functional SDF-1 gene in mice led to the absence of the attractive activity for the embryonic EGL cells in the meninges. Our work therefore shows that the predominant, if not the sole, attractant in the meninges for the EGL cells is the chemokine SDF-1. Our studies with purified SDF-1 protein (Fig. 2i) indicate that it attracts the embryonic EGL cells directly rather than attracting glial fibers or regulating the expression of other direct attractants in the meninges. This evidence for chemokine guidance of neuronal migration further supports our recent proposal of a fundamental conservation of guidance mechanisms for cells as distinct as neurons and leukocytes12,13. SDF-1 is expressed in the meninges covering the entire CNS, and it is possible that it also regulates the development or function of other parts of the CNS.
Acknowledgments
We are grateful to the US National Institutes of Health (NIH), the Klingenstein foundation, the John Merck fund and the Leukemia Society of America for support (to Y.R. and J.Y.W.).
Footnotes
Note: Supplementary material is available on the Nature Neuroscience website.
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Tashiro K, et al. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 2.Nagasawa T, et al. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 3.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 4.Ma Q, et al. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tachibana K, et al. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 6.Asensio VC, Campbell IL. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 7.Mennicken F, Maki R, de Souza EB, Quirion R. Trends Pharmacol Sci. 1999;20:73–78. doi: 10.1016/s0165-6147(99)01308-5. [DOI] [PubMed] [Google Scholar]
- 8.Lu Q, Sun E, Klein RS, Flanagan JG. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 9.Jazin EE, Soderstrom S, Ebendal T, Larhammar D. J Neuroimmunol. 1997;79:148–154. doi: 10.1016/s0165-5728(97)00117-3. [DOI] [PubMed] [Google Scholar]
- 10.Song HJ, Ming GL, Poo MM. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 11.Poznansky MC, et al. Nat Med. 2000;6:543–548. doi: 10.1038/75022. [DOI] [PubMed] [Google Scholar]
- 12.Wu JY, et al. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu W, et al. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]


